Abstract
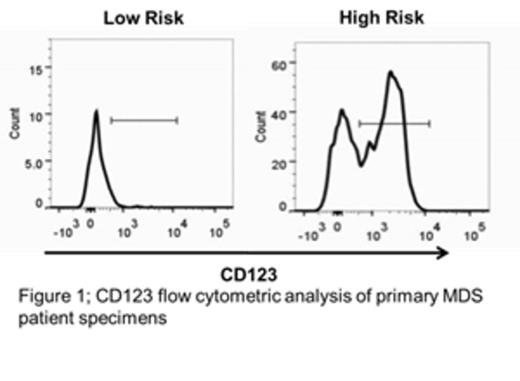
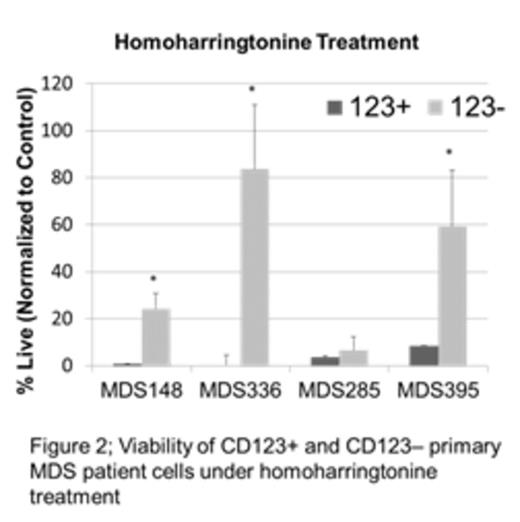
Myelodysplastic syndrome (MDS) is a malignant disorder of myeloid hematopoietic cells, resulting in ineffective hematopoiesis leading to bone marrow dysfunction and failure, and frequently evolves to acute myelogenous leukemia (AML). While chemotherapy can on occasion induce transient remissions, the disease is typically progressive, with the vast majority dying of MDS complications or AML. While MDS is thought to arise from a malignant stem cell population, the properties of such cells are not well understood. Thus, we performed studies to better characterize candidate MDS stem cells and allow insights into the development of novel stem cell directed targeted therapies. One line of investigation examined the cytokine receptor CD123, which we have previously shown to be a marker of AML stem cells. Analysis of primary human specimens demonstrates that CD123 is not expressed in the normal stem cell population (phenotypically identified as CD34+/CD38-) or in stem cells(CD34+/CD38-) from low risk (<5% blasts and/or ≤ intermediate risk IPSS-R) MDS patients, but is readily detected in high-risk patients (>5% blasts and/or ≥ high risk IPSS-R) as shown in figure 1. These data suggest that up-regulation of CD123 may be a hallmark of pathogenesis as MDS progresses toward AML. To better understand the properties of CD123+ vs. CD123- stem cells, we performed global expression studies (RNA-seq) on sorted primary cell populations derived from high-risk MDS patients. The data indicate strong up-regulation of protein synthesis machinery, which indicates a major change in underlying cellular physiology and metabolism. Increased protein synthesis in CD123+ MDS stem cells was verified using the fluorescent protein substrate OP-puro, which identifies newly synthesized polypeptides. We found a ~13 fold difference in OP-puro staining of the CD123+ MDS stem cells versus CD123- cells. Based on these findings, we pursued two related lines of investigation to develop targeted therapies against MDS stem cells. First, we tested the protein synthesis inhibitors anisomycin and homoharringtonine for their ability to target the candidate MDS stem cell population; both agents demonstrated highly selective eradication of CD123+ stem cells, with a significant differential toxicity observed in multiple samples (up to an 80-fold difference in viability after treatment) as shown in figure 2. Second, because protein synthesis inhibition is known to rapidly down-regulate Bcl-2, we tested a selective Bcl-2 inhibitor. This agent also selectively eradicated CD123+ MDS stem cells in 4 of 10 patient samples tested. Finally, analysis of protein synthesis inhibitors or a Bcl-2 inhibitor in combination with the hypomethylating agent 5-azacytidine demonstrated potent efficacy in targeting the CD123+ stem cell population with greater then additive toxicity when compared to single agent treatment. We found significant differential sensitivity in the CD123+ population to the Bcl-2 inhibitor + 5-azacytidine in 9 out of 10 in samples tested and found significant toxicity to the combination of anisomycin or homoharringtonine with 5-azacytidine in 4 out of 4 samples. Taken together, these data show that we have identified two novel pharmacological approaches that may effectively target the MDS stem cell population. Further, these agents may function well in conjunction with commonly used agents used in the treatment of MDS.
Pollyea:GlycoMimetics: Other: Member of data safety monitoring board; Pfizer: Consultancy; Ariad: Consultancy; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.