Abstract
Background: The introduction of newer agents, such as lenalidomide (LEN) and bortezomib (BORT), has improved survival outcomes for pts with RRMM (Kumar et al Leukemia, 2014). However, overall survival (OS) in pts who have failed or progressed on treatment (Tx) with newer agents is short and there are few additional treatment options available (Kumar et al Leukemia, 2012). POM is a distinct immunomodulatory agent with tumoricidal and immunoregulatory effects approved in combination with DEX for the Tx of pts with RRMM in the US and EU with ≥ 2 prior Txs, including LEN and BORT. Pivotal trials with POM + LoDEX have demonstrated greater survival benefits when compared with high-dose DEX (MM-003; San Miguel et al Lancet Oncol, 2013) or POM alone (MM-002; Richardson et al Blood, 2014). The STRATUS trial (MM-010; ClinicalTrials.gov: NCT01712789; EudraCT: 2012-001888-78) is a single-arm, open-label, phase 3b study being conducted in 19 countries across Europe designed to further evaluate safety and efficacy of POM + LoDEX in RRMM. Here, we present an updated analysis of safety and efficacy.
Methods: Pts with refractory or relapsed and refractory disease (progressive disease [PD] on or within 60 days of last prior Tx), Tx failure with BORT and LEN, and adequate prior alkylator therapy were eligible. Pts with the following laboratory values were excluded: absolute neutrophil count < 800/μL; platelets < 75,000 or < 30,000/μL for pts with < 50% or ≥ 50% of bone marrow nucleated cells as plasma cells, respectively; creatinine clearance < 45 mL/min; hemoglobin < 8 g/dL; and peripheral neuropathy (PN) grade (Gr) ≥ 2. POM 4 mg was administered days 1-21 of a 28-day cycle in combination with LoDEX 40 mg/day (20 mg for pts aged > 75 yrs) on days 1, 8, 15, and 22 until PD or unacceptable toxicity. Thromboprophylaxis was required for all pts.Follow up continued for subsequent Tx, OS, and second primary malignancy until 5 yrs post enrollment. Safety was the primary end point, and key secondary end points included overall response rate (ORR) (≥ partial response), duration of response (DOR), progression-free survival (PFS), OS, and POM exposure.
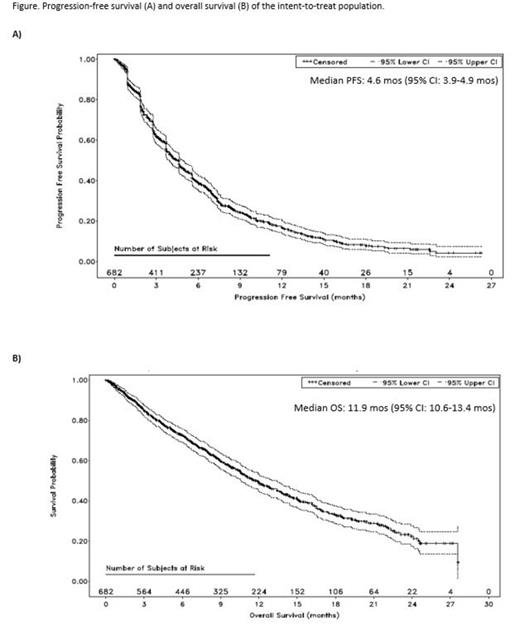
Results: A total of 682 pts were enrolled. The median age was 66 yrs (range, 37-88 yrs), and the median time since diagnosis was 5.3 yrs (range, 0.5-28.1 yrs). Pts were heavily pretreated with a median of 5 prior regimens (range, 2-18). Most pts were refractory to LEN (96%), BORT (84%), or LEN and BORT (80%). As of May 4, 2015, the median follow up was 16.8 mos and the median duration of Tx was 4.9 mos. In the 676 pts receiving POM + LoDEX, the most frequent Gr 3/4 treatment-emergent adverse events (TEAEs) were hematologic events (neutropenia [49.7%], anemia [33.0%], and thrombocytopenia [24.1%]. The most common Gr 3/4 nonhematologic toxicities were pneumonia (12.9%), fatigue (5.9%), and hypercalcemia (5.0%). Incidences of Gr 3/4 venous thromboembolism (deep vein thrombosis and pulmonary embolism) and PN were low (1.6% and 1.5%, respectively). Dose reductions, interruptions, and discontinuations of POM due to TEAEs were required in 22.0%, 66.3%, and 6.1% of pts; respectively. The ORR was 32.6%, with 0.6%, 7.6%, and 24.3% of pts achieving a complete response, very good partial response, and partial response, respectively. The median DOR was 7.4 mos. Median PFS and OS were 4.6 mos and 11.9 mos, respectively (Figure).
Conclusions: The updated safety and efficacy results of STRATUS, the largest study of POM + LoDEX in a heavily pretreated RRMM population, were in line with results from the pivotal trials demonstrating that POM + LoDEX is effective and well tolerated. This analysis confirms that POM + LoDEX is a standard of care for pts with RRMM.
Dimopoulos:Janssen: Honoraria; Janssen-Cilag: Honoraria; Genesis: Honoraria; Celgene: Honoraria; Novartis: Honoraria; Onyx: Honoraria; Amgen: Honoraria. Palumbo:Celgene, Millennium Pharmaceuticals, Amgen, Bristol-Myers Squibb, Genmab, Janssen-Cilag, Onyx Pharmaceuticals: Consultancy, Honoraria; Novartis, Sanofi Aventis: Honoraria. Cavo:Janssen-Cilag, Celgene, Amgen, BMS: Honoraria. Delforge:Novartis: Honoraria; Celgene Corporation: Honoraria; Janssen: Honoraria; Amgen: Honoraria. Weisel:Amgen: Consultancy, Honoraria, Other: Travel Support; Janssen Pharmaceuticals: Consultancy, Honoraria, Other: Travel Support, Research Funding; Celgene: Consultancy, Honoraria, Other: Travel Support, Research Funding; Novartis: Other: Travel Support; BMS: Consultancy, Honoraria, Other: Travel Support; Onyx: Consultancy, Honoraria; Noxxon: Consultancy. Di Raimondo:Celgene Corporation: Research Funding, Speakers Bureau. Simcock:Celgene Corporation: Employment. Miller:Celgene Corporation: Employment, Equity Ownership. Slaughter:Celgene Corporation: Employment, Equity Ownership. Peluso:Celgene Corporation: Employment. Sternas:Celgene Corporation: Employment, Equity Ownership. Zaki:Celgene Corporation: Employment, Equity Ownership. Moreau:Celgene: Honoraria, Other: Adboard; Amgen: Other: Adboard; Janssen: Honoraria, Other: Adboard; Takeda: Honoraria, Other: Adboard; Novartis: Honoraria, Other: Adboard.
Author notes
Asterisk with author names denotes non-ASH members.