Abstract
Background: Low-molecular-weight heparin (LMWH) is considered to be an anticoagulation therapy for the treatment of cancer-associated thrombosis. The duration of treatment is recommended to maintain at least 3-6 months after the diagnosis. However, the data on continuing LMWH treatment beyond six months remains unclear.
Methods: Consecutive cancer-associated thrombosis patients who were enrolled in RIETE Registry were evaluated. We systematically selected the patients who completed treatment with LMWH for 6 months. The patients were divided into two groups whether they continued to receive LMWH or switched to warfarin. The main outcomes were recurrent venous thromboembolism (VTE), major bleeding and total bleeding. Survival curves were generated using Kaplan-Meier method and the curves were compared using the log-rank test. Hazard ratio (HR) with corresponding 95% confidence interval (CI) were calculated using Cox-proportional hazard (PH) model.
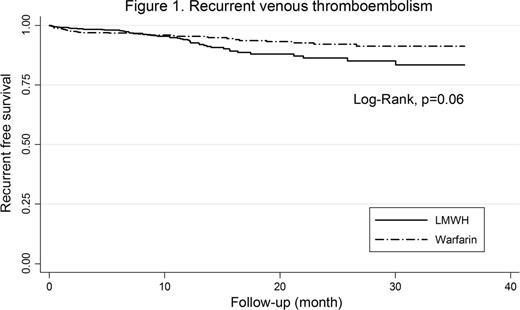
Outcomes: of the 1,502 eligible patients who completed 6-month anticoagulant therapy, 763 patients continued to received LMWH and 739 switched to warfarin. There was no significant difference in terms of recurrent VTE between two study groups (hazard ratio [HR] 0.67, 95% confidence interval [CI]; 0.44-1.02, p=0.06), Figure 1. The cumulative incidence of major bleeding was 2.6% in LMWH group and 2.7% in warfarin group (HR 1.05, 95%CI; 0.79-1.55, p=0.79), Figure 2. The cumulative incidence of total bleeding was 6.7% in LMWH group and 7.0% in warfarin group (HR 0.92, 95%CI; 0.62-1.37, p=0.70).
Conclusions: In patients with cancer-associated thrombosis who completed 6-month of anticoagulation therapy, switching to warfarin is not associated with increase in recurrent VTE, major bleeding or total bleeding when compared to continuing LMWH. Warfarin is an acceptable alternative anticoagulant in cancer-associated thrombosis patients who do not tolerate long-term treatment with LMWH.
Monreal:Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees; sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; boehringer: Consultancy, Membership on an entity's Board of Directors or advisory committees; daichii: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract