Abstract
Background: Hepatic SOS is a serious complication after allogeneic HSCT. It has been reported elsewhere that in the period between 1979-2007, the overall mean incidence of hepatic SOS was 13.7% (95%CI:13-15). While prophylactic procedures are widely used, there is no uniform consensus on the optimal strategy for the prevention of this disease. Low dose UFH have been used but its benefit in the prevention of SOS and the potential associated risk of serious bleeding complications have not been fully established.
Methods: We evaluated 730 adult allograft recipients transplanted 01/2003-12/2013 for hematologic malignancies [401 (55%) acute leukemia/ MDS, 235 (32%) lymphoma, 64 (9%) multiple myeloma, 30 (4%) CML/myeloproliferative disorder]. The majority received myeloablative conditioning (n = 466, 65%) with either total body irradiation-based (n = 243) or busulfan-based (n = 233), followed by non-myeloablative conditioning (n = 143, 19%) and reduced intensity (RIC) (n = 111, 15%). Approximately half of the patients had T-cell depleted (TCD) grafts (402, 55%). All patients had SOS prophylaxis with low dose heparin 100 units/kg given as continuous IV over 24 hours from admission to day +21 or engraftment. Prior allogeneic HSCT, non-malignant or refractory disease, dual prophylaxis with UFH/ursodiol or therapeutic anticoagulation at the time of allograft admission were all excluded.
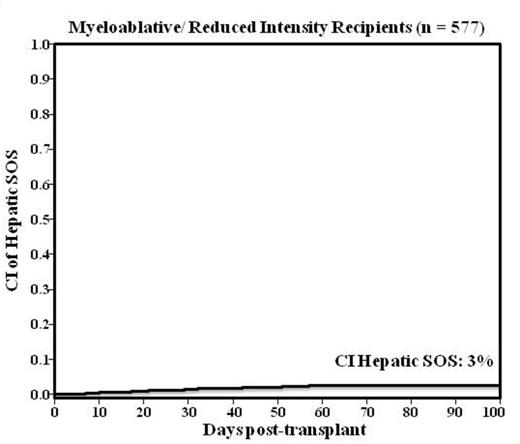
Results: The median age was 51 (range 21-65) years, and 415 (56%) were male. During the 10 year study period only 15 patients developed hepatic SOS with a day 100 incidence of 2% (95%CI:1-3). When evaluated by conditioning intensity, myeloablative and reduced intensity conditioning recipients had a day 100 incidence of 3% (95%CI:2-4) (Figure) whereas none of the NMA recipients developed SOS. Univariate analysis showed that age, HLA-match, previous hepatitis B or C exposure did not influence SOS development (Table). There was a significantly higher incidence of hepatic SOS in recipients of unmodified (3%) versus TCD grafts (1%), p= 0.025. The median time to SOS onset was 29 days (range 5-57). Six patients received supportive care only whereas 9 had defibrotide therapy. Of those, 7 patients developed severe SOS resulting in multi-organ failure (5 supportive care, 2 defibrotide). Four of 15 patients diagnosed with hepatic SOS are alive at last follow up (7 deaths attributed to SOS). Bleeding complications occurred in 26/730 patients (4%) of which only 4 (0.5%) patients had significant grade 3-4 bleeding. The day 100 grade II-IV and III-IV acute liver GVHD is 2% (95%CI:1-3) and 0.5% (95%CI:0.2-1), respectively. The incidence of day 100 TRM was 9% (95%CI:7-11) for the entire cohort of patients.
Conclusion: To our knowledge, this is the largest analysis evaluating the use of low dose UFH for the prevention of hepatic SOS. We demonstrated that UFH prophylaxis is associated with a low incidence of hepatic SOS, is well tolerated, and severe bleeding complications are uncommon. Low dose UFH may be consider as a strategy for the prevention of hepatic SOS in selected patients. Further prospective studies comparing UFH to other hepatic SOS prophylaxis methods are warranted.
Univariate analysis of variables potentially associated with day 100 SOS.
| Variable . | (95% CI) . | p-value . |
|---|---|---|
| Age (years) | 0.745 | |
| < 40 | 2% (1-6) | |
| ≥ 40 | 2% (1-3) | |
| HLA-match | 0.857 | |
| 8/8 | 2% (1-4) | |
| ≤ 7/8 | 2% (1-5) | |
| Graft | 0.025 | |
| TCD | 1% (0.3-2) | |
| Unmodified | 3% (2-6) | |
| Conditioning regimen | 0.054 | |
| Myeloablative/RIC | 3% (2-4) | |
| Non-Myeloablative | 0 (NA) | |
| Myeloblative conditioning type | 0.353 | |
| Busulfan-based | 2% (1-5) | |
| TBI-based | 3% (2-6) | |
| Hepatitis B & C Serologies | 0.089 | |
| Negative | 2% (1-3) | |
| Positive | 8% (0.4-30) |
| Variable . | (95% CI) . | p-value . |
|---|---|---|
| Age (years) | 0.745 | |
| < 40 | 2% (1-6) | |
| ≥ 40 | 2% (1-3) | |
| HLA-match | 0.857 | |
| 8/8 | 2% (1-4) | |
| ≤ 7/8 | 2% (1-5) | |
| Graft | 0.025 | |
| TCD | 1% (0.3-2) | |
| Unmodified | 3% (2-6) | |
| Conditioning regimen | 0.054 | |
| Myeloablative/RIC | 3% (2-4) | |
| Non-Myeloablative | 0 (NA) | |
| Myeloblative conditioning type | 0.353 | |
| Busulfan-based | 2% (1-5) | |
| TBI-based | 3% (2-6) | |
| Hepatitis B & C Serologies | 0.089 | |
| Negative | 2% (1-3) | |
| Positive | 8% (0.4-30) |
Bhatt:Spectrum: Consultancy. Off Label Use: Use of low dose unfractionated heparin for the prevention of hepatic SOS. van den Brink:Boehringer Ingelheim: Consultancy, Other: Advisory board attendee; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Regeneron: Honoraria; Merck: Honoraria; Tobira Therapeutics: Other: Advisory board attendee. Giralt:TAKEDA: Consultancy, Honoraria, Research Funding; AMGEN: Consultancy, Research Funding; JAZZ: Consultancy, Honoraria, Research Funding, Speakers Bureau; SANOFI: Consultancy, Honoraria, Research Funding; CELGENE: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.