Abstract
Introduction: Genetic modification of T cells with chimeric antigen receptor (CAR) has emerged with astonishing treatment outcomes for B cell malignancies. Clinical trials of CAR-T therapy demonstrated toxicities such as hypogammaglobulinemia due to B cell aplasia or hemophagocytic syndrome after overactivation of CAR-T cells. These toxicities are considered as major drawbacks for broader application of CAR-T therapy. To overcome these serious adverse events, further modification of CAR-T technology to control CAR expression arbitrary is needed. Therefore we aimed to develop inducible CAR expressing T cells based on tetracycline-regulation system.
Methods: We developed a novel inducible CD19CAR system by infusing anti-CD19-CD3z-CD28-tEGFR into pRetroX-TetOne vector (Tet-19CAR). By using Tet-19CAR transduced SUPT1 (T cell line), expression and disappearance kinetics of CAR were determined. We also retrovirally transduced Tet-19CAR into human CD8+ T cells, and achieved more than 90% purity of CAR positive T cells after a selection with anti-EGFR mAb. These CAR-T cells were again expanded with anti-CD3/28 beads and used in 51 Cr release assay, coculture assay, cytokine release assay and T cell proliferation assay. Regarding coculture assay, CD19 transduced K562-CD19 (K562-CD19) was labeled with 0.1 nM CFSE and plated with CAR-T cells at a ratio of 1:1 without IL-2 supplementation and incubated for 96 hours. Finally we examined this system in NOG mice. We injected 0.5 x 106 Raji-ffluc (fire-fly luciferase) followed by 5.0 x 106 CAR-T cells from the tail vein, then we evaluated the tumor flux by in vivo imaging system on days 7, 14, 21, and 30.
Results: With more than 100 ng/mL of Doxycycline (Dox), CD19CAR was successfully expressed on both of SUPT1 and CD8+ T cells. For maximum and minimum expression, 24 and 72 hours were needed after addition and discontinuation of Dox, respectively. To determine the cytotoxicity of Tet-19CAR-T cells according to presence or absence of Dox, we performed 51 Cr release assay and coculture assay against K562-CD19. In the presence of Dox, Tet-19CAR showed an equivalent lytic activity to conventional CD19CAR-T cells (c19CAR). In contrast, Tet-19CAR without Dox exhibited significantly lower cytotoxicity against CD19+ target cells. (Dox (-) Tet-19CAR, Dox (+) Tet-19CAR and c19CAR: 14.0±4.0%, 38.0±4.0% and 37.0±2.0% at an E:T ratio = 10:1, respectively). In the coculture assay, Tet-19CAR with Dox eradiated K562-CD19, while they failed to suppress the target cells without Dox.
In the intracellular IFN-g assay against K562-CD19, a similar proportion of responder was IFN-g + in Tet-19CAR with Dox and c19CAR. On the other hand, a significantly low proportion of IFN-g + cells were observed in Tet-19CAR without Dox. (Dox (-) Tet-19CAR, 1.0%±0%, Dox (+) Tet-19CAR, 19.1%±6.0% and c19CAR 21.5%±4.0%, respectively)
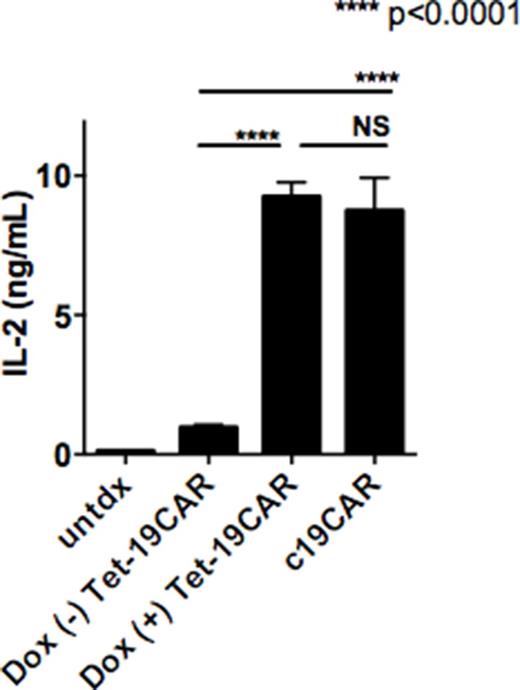
Similar to intracellular IFN-g assay, ELISA revealed that Tet-19CAR with Dox and c19CAR produced IL-2 and IFN-g equally well. However, Tet-19CAR without Dox hardly did. [IL-2 (ng/ml): Dox (-) Tet-19CAR, 1.00±0.060, Dox (+) Tet-19CAR, 9.25±0.30 and c19CAR 8.75±0.68; IFN-g (ng/ml): 2.32±1.24, 57.96±6.95 and 62.42±5.95] (Fig).
We next analyzed CAR-T cell proliferation upon stimulation with K562-CD19 over 96 hours. Tet-19CAR with Dox showed 6-7 fold expansion, whereas Tet-19CAR without Dox failed to proliferate.
Regarding in vivo model, the mice treated with c19CAR or Tet-19CAR with Dox showed significantly low tumor flux but the mice treated with Tet-19CAR without Dox showed higher tumor burden at day 21 of CAR-T cell infusion [Photons/sec: Dox (-) Tet-19CAR, 2.5 x 1010, Dox (+) Tet-19CAR, 6.4 x 108 and c19CAR, 8.4 x 108 ].
Conclusions: We generated tetracycline-inducible CAR-T cells and successfully controlled the CAR expression with Dox administration. Tet-19CAR without Dox still demonstrated some CD19CAR expression and subsequent cytotoxicity against CD19 positive cells. Nonetheless the CAR expression level of Tet-19CAR without Dox was lower than the threshold for exhibiting positive responses in the function assays such as cytokine production and proliferation. This phenomenon was also confirmed in the xenograft model. To regulate CAR expression more precisely and pursue clinical translations in combinations with other CARs, further efforts are needed to reduce any leaky CAR expression by modification of the system.
Kiyoi:Pfizer Inc.: Research Funding; Eisai Co., Ltd.: Research Funding; Yakult Honsha Co.,Ltd.: Research Funding; Alexion Pharmaceuticals: Research Funding; MSD K.K.: Research Funding; Takeda Pharmaceutical Co., Ltd.: Research Funding; Taisho Toyama Pharmaceutical Co., Ltd.: Research Funding; Teijin Ltd.: Research Funding; Astellas Pharma Inc.: Consultancy, Research Funding; Japan Blood Products Organization: Research Funding; Nippon Shinyaku Co., Ltd.: Research Funding; FUJIFILM RI Pharma Co.,Ltd.: Research Funding; Nippon Boehringer Ingelheim Co., Ltd.: Research Funding; FUJIFILM Corporation: Patents & Royalties, Research Funding; Zenyaku Kogyo Co., Ltd.: Research Funding; Sumitomo Dainippon Pharma Co., Ltd.: Research Funding; Kyowa Hakko Kirin Co., Ltd.: Consultancy, Research Funding; Bristol-Myers Squibb: Research Funding; Chugai Pharmaceutical Co., Ltd.: Research Funding; Novartis Pharma K.K.: Research Funding; Mochida Pharmaceutical Co., Ltd.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.