Abstract
Introduction
A study of diagnostic test accuracy compares a single index test to a gold standard to determine status of disease. The observed accuracy of a test varies among patient subgroups and is sensitive to bias. To achieve reliable estimates of diagnostic accuracy, an appropriate study design in a clinically relevant population is warranted. Recently a review was published about the evolution of the bleeding assessment tool (BAT) in diagnosing patients with mild bleeding disorders (MBD) (Rydz et al. J Thromb Haemost 2012). Many validation studies have been done. However, a critical appraisal addressing the quality of these validation studies is lacking.
Objective
We performed a systematic review to determine the quality and applicability of studies assessing the diagnostic utility of the BAT for MBD among clinic based cohorts.
Methods
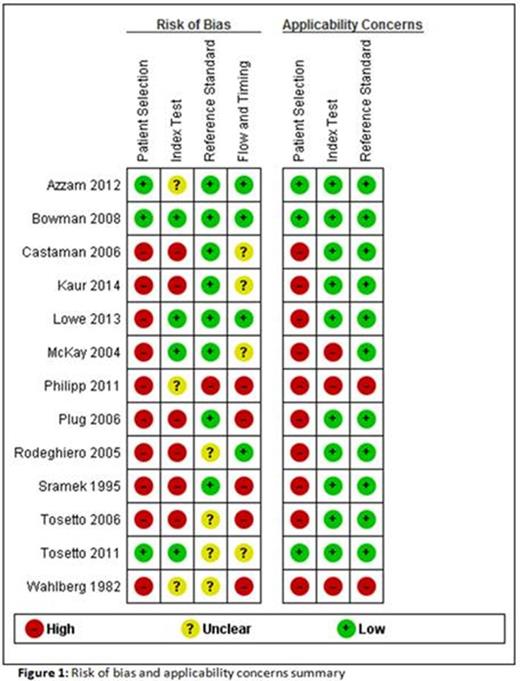
The literature search was conducted using the electronic database PubMed. The final search date was March 2, 2015. The search terms: 'bleeding disorder OR bleeding tendency' AND 'bleeding questionnaire' were used. All studies assessing the diagnostic accuracy of bleeding questionnaires in identifying adults (age > 18 years) with MBD were considered eligible, irrespective of study design or used reference standard. The methodological quality and applicability of each included study was assessed using a Quality Assessment of Diagnostic studies-2 (QUADAS-2) tool. This tool consists of four domains specific for patient selection, index test, reference standard and participant flow. For each domain bias was assessed using signaling questions, for the first three domains applicability was assessed.
Results
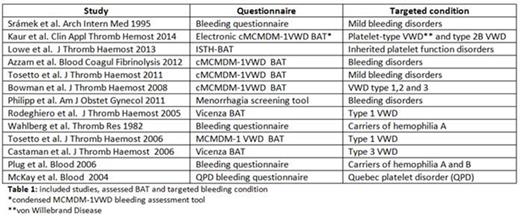
The search yielded 530 citations, from which 35 possible relevant full-text studies were identified. Twenty-two studies were excluded, reasons for exclusion were: letter to the editor, validation of questionnaire combined with laboratory results and primary care population. Table 1 shows the 13 included studies, the assessed BAT and the targeted bleeding condition. Risk of bias and applicability concerns are summarized in figure 1. In 77% of the studies there was a high risk of bias for patient selection and applicability concerns. Many studies used a case control design, comparing patients with a known bleeding disorder with healthy controls. This leads to spectrum bias and might generate higher estimates of sensitivity and specificity (Rutjes et al. Clin Chem 2005). In 46% there was a high risk of bias for index test due to the use of a self-administered questionnaire or because the person conducting the questionnaire was aware of the diagnosis. This leads to observer bias caused by better awareness and over-reporting of bleeding symptoms. Finally, there was high risk of bias in study flow in 38% of the studies. These studies included symptoms after diagnosis of the bleeding disorder. Since bleeding disorders are managed by interventions to prevent bleeding, underestimation of the bleeding symptoms may occur.
Conclusion
This review highlights the difficulties and advantages of the BAT validation studies. It provides the ability for medical practitioners to apply the BAT with full awareness of its restrictions and benefits. With the evaluation of the risks of bias in the included studies we highlighted limitations, especially in method of patient selection and use of index test, that future studies preferably should try to avoid.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.