Abstract
Introduction:
Anemia is the most common hematological abnormality in patients with cancer and hematological malignancies, and is associated with poor prognosis and outcomes that have a detrimental impact on the patient's condition and quality of life (QOL). Erythropoiesis-stimulating agents (ESA) represent a good treatment option in order to increase the hemoglobin level in patients with anemia. Anemia can also be treated by red blood cell transfusion, but this has a transient effect and is associated with risks such as exposure to infectious agents, iron overload, or transfusion-related acute lung injury. ESA also have safety concerns, including the established increased risk of venous thromboembolic events. However, they are currently the only therapeutic alternative to transfusions. We performed a prospective observational study in patients undergoing allogeneic hematopoietic stem cell transplantation (allo-HSCT) for hematological malignancies, with the primary objective of evaluating the effect of a new ESA biosimilar, epoetin zeta (Hospira) on patient QOL. Secondary objectives included hemoglobin (Hb) and platelet (Pt) recovery, safety, overall survival (OS) and relapse incidence. Results of this study were compared to two reference populations, one receiving epoetin beta (Roche) and one control group not treated with ESA. Here, we present preliminary results for the secondary objectives.
Materials and methods:
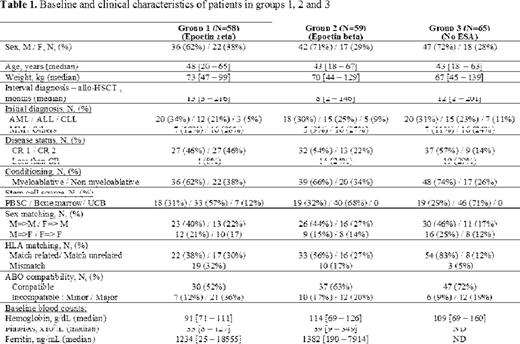
The study included adult patients with Hb level ≤11g/dl occurring after all types of allo-HSCT for any hematological disease (Table 1). Epoetin zeta (30,000 IU) was administered s.c. once per week for up to 6 months, and Hb levels were monitored weekly. Injections were stopped once the Hb level reached 12g/dl without transfusion. If after 4 injections, no improvement was observed, doses were doubled, and if after 8 injections, no improvement was observed, the patient was withdrawn from the study. The QOL was measured at baseline and at 1, 2, 3 and 6 months by the Functional Assessment of Cancer Therapy-Anemia (FACT-An) scale. Epoetin zeta responders were defined as having Hb level ≥12g/dl (complete response, CR) or a ≥2g/dl increase (partial response, PR) compared with baseline value, in the absence of transfusion. Patients receiving epoetin zeta (group 1) were compared to a similar population receiving epoetin beta with the same procedures (group 2) and to a matched population not treated with ESA (group 3), taking into account the following variables: sex, age, diagnosis, disease status at allo-HSCT, conditioning regimen and HSC source.
Results:
Between December 2011 and September 2014, 58 patients (from 168 screened) were included in group 1, and compared to 59 patients in group 2 and 65 patients in group 3. The main exclusion criteria were ESA contra-indication and patient refusal. Patients in group 1 had lower Hb baseline levels compared to group 2; patient characteristics for each group are summarized in Table 1. The median number of injections/patient was 10 (range: 6-14) in group 1 and 8 (range: 2-28) in group 2. The cumulative incidence of CR was 80% in group 1 and 71% in group 2. The median time to achieve CR was 48 days (range: 35-70) in group 1, and 39 days (range: 14-180) in group 2. Eight patients withdrew due to ESA inefficacy in group 1 and 8 in group 2. Adverse events were all thromboembolic: 2 events in group 1 and 5 events in group 2, compared to 2 events in group 3 (p=0.34). The multivariate analysis studying different confounding factors on the cumulative incidence of CR showed a significant positive impact of younger age (p=0.001), and a negative impact of being female or having major ABO incompatibility. We did not find any significant difference in terms of OS and relapse rate between the 3 groups.
Conclusion:
We describe here, for the first time, preliminary data for ESA biosimilar epoetin zeta (Hospira) in allo-HSCT patients showing comparable efficacy and safety to an existing ESA, epoetin beta (Roche) with no impact on OS and relapse incidence, compared to a control group. The QOL and transfusion evaluations as well as a cost-effectiveness study are ongoing and results will be presented.
Nicolini:Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Ariad Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.