Abstract
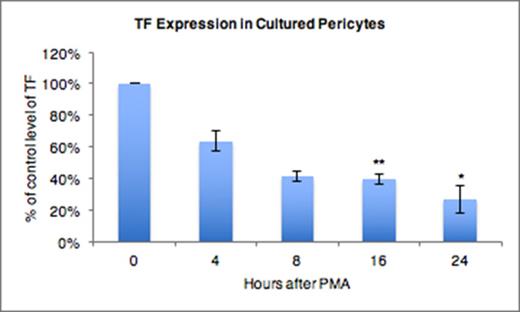
Tissue factor (TF) is a transmembrane protein in the type II cytokine receptor family that serves as a cofactor for factor VIIa activity, and is essential for normal initiation of hemostasis. Its expression is generally upregulated in the settings of inflammation, immune activation, tissue injury and malignancy. TF has biological functions in addition to its role hemostasis. In malignancy, high expression of TF by tumor cells promotes local angiogenesis. By contrast, we have reported that expression of perivascular TF is downregulated within 24 hours after wounding, and remains low around angiogenic vessels during physiologic wound healing. The mechanism(s) of physiologic downregulation of TF, and its role in physiologic angiogenesis have not been previously characterized. Therefore, the objective of this study was to identify mechanisms contributing to TF downregulation in primary human pericytes. We determined that loss of TF in human pericytes can be recapitulated in vitro by treating pericyte cultures with phorbol 12-myristate 13-acetate (PMA). The time course and degree of TF downregulation over 24 hours, as shown in the figure, is similar to that observed within the first 24 hours in vivo. Since PMA is a potent activator of Protein Kinase C (PKC), we determined the role of PKC in PMA-mediated TF loss by pretreating pericytes with the broad spectrum PKC inhibitor, Go 6983, prior to stimulation with PMA. PKC inhibition eliminated PMA-induced TF loss, supporting the conclusion that the PMA effect occurs through a PKC-dependent mechanism. PKCa has been shown to induce phosphorylation of the TF cytoplasmic tail, which results in budding of TF-rich microvesicles from the plasma membrane. PMA also triggers activation of the cell-surface protease ADAM17. Based on domain homology with other targets, ADAM17 could potentially cleave and release the extracellular domain of TF from the cell surface. To determine if TF loss occurs through its release from the cell by either mechanism we utilized biotin labeling of surface TF, streptavidin pull down, and western blot analysis to assess trafficking of TF into the culture media. Our results show that surface TF significantly decreases within 2 hours of PMA treatment. However, we failed to detect biotinylated TF in the culture media. Isolation of microvesicles from the media followed by western blotting for TF showed that pericytes do release TF-associated microvesicles at a basal rate, but this rate is not affected by PMA. Taken together, these data show that PMA induces downregulation of TF in primary human pericytes through a PKC-dependent mechanism, and that this mechanism does not stimulate release of TF from the cell in either microvesicles or by proteolytic cleavage of the TF extracellular domain. Our working hypothesis is that PMA triggers internalization of surface TF via activation of protein kinase C, leading to its subsequent degradation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.