Abstract
Background
Immune thrombocytopenia (ITP) is a disease caused by autoimmune destruction and impaired production of platelets. It can be primary or secondary in origin. It manifests as isolated thrombocytopenia with platelet counts less than 100 x109/L. Therapy is typically initiated when the platelet counts fall to less than 20 to 30 x 109/L in asymptomatic patients to reduce the risk of bleeding. ITP that persists for greater than 12 months is classified as chronic and ITP that endures despite splenectomy is defined as refractory. Most therapeutic approaches, such as corticosteroids, target the pathologic platelet destruction. Thrombopoietin (Tpo) mimetics, on the other hand, enhance platelet production via stimulation of the Tpo receptor on megakaryocytes. Studies suggest that Tpo mimetics increase reticulin deposition in the bone marrow. Tpo mimetics are postulated to promote reticulin deposition through stimulation of cytokine production by megakaryocytes, including transforming growth factor beta. There are reports of myelofibrosis, with abnormal deposition of collagen and reticulin in bone marrow trephine biopsies of ITP patients treated with these agents. Tpo mimetic induced myelofibrosis however, is described as reversible upon cessation of Tpo based therapy in most reports. Here we present a case of myelofibrosis induced by sequential therapy with two high dose TPO mimetics, which was not reversed six months after discontinuation of the medications.
Case Report
An 87-year-old woman with chronic ITP resistant to multiple lines of therapy (prednisone, dexamethasone, rituximab, dapsone, mycofenylate mofetil, and splenectomy) was treated consecutively with two thrombopoietin (Tpo) mimetics, romiplostim and then eltrombopag. Bone marrow evaluation at diagnosis, prior to treatment with Tpo mimetics, showed megakaryocyte hyperplasia without evidence of fibrosis. There was no definitive evidence of an underlying clonal hematologic disorder.. The patient's course was complicated by gastrointestinal and intracranial hemorrhage. Tpo mimetics were introduced as fourth line therapy. ITP response to romiplostim and then to eltrombopag was initially encouraging but rapidly fleeting.The peak dose of romiplostim used was 10 mcg/kg/week and eltrombopag was used at a dose of 75 mg per day. While on eltrombopag and within six months of romiplostim, therapy, she developed persistent left-shifted leukocytosis and a peripheral blood film showing leukoerythroblastosis and dacrocytic erythrocytes. This prompted a bone marrow evaluation that confirmed myelofibrosis, with a dense and diffuse increase in reticulin content and focal bundles of collagen (MF 2 of 3). There was associated marrow hypercellularity, increased numbers of enlarged megakaryocytes, clustered megakaryocyte topography and extensive bone resorption and remodelling Eltrombopag was promptly discontinued and peripheral blood leukoerythroblastic picture improved. Repeat bone marrow evaluation 6 months after stopping therapy showed partial reversal of the abnormalities seen in the previous marrow, including a reduction in the extent of reticulin fibrosis without any evidence of collagen fibrosis (MF 1-2 of 3). These changes are shown in Figure 1.
Discussion
Chronic, refractory ITP is a challenging syndrome to treat, and the Tpo mimetics have emerged as an important therapeutic option for patients who have failed traditional therapies. Clinical experience with the relatively novel Tpo mimetic drug class is maturing as we obtain longitudinal data and reports about adverse effects like myelofibrosis. While the myelofibrosis in our patient improved upon cessation of Tpo mimetic therapy, it did not resolve entirely and persisted 6 months after stopping therapy. We hypothesize that the sequential use of high dose Tpo mimetic therapy may have contributed to the development and persistence of myelofibrosis in this patient. This report adds to the body of evidence suggesting that myelofibrosis is a serious and, potentially chronic, adverse effect of treatment with Tpo mimetics.
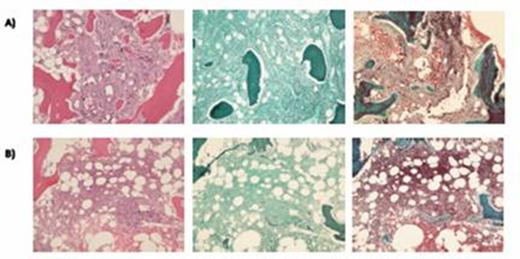
TPO mimetic treatment and B) Grade 1-2 of 3 Myelofibrosis after cessation of TPO mimetic.
Semiquantitative grading is based on the 2008 WHO classification.
Images from left to right represent hematoxylin and
eosin, reticulin, and trichrome stains.
Bone marrow pathology slides comparing A) Grade 2 of 3 Myelofibrosis during
Bone marrow pathology slides comparing A) Grade 2 of 3 Myelofibrosis during
Sholzberg:CSL Behring: Honoraria, Research Funding; Baxalta: Honoraria, Research Funding; Amgen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.