Abstract
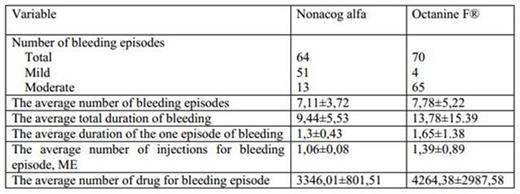
The efficiency and safety of a new Russian recombinant factor IX - FIX (nonacog alpha, Innonafactor®, GENERIUM) in prevention of bleeding episodes in patients with severe and moderate hemophilia B (part 1 of phase II-III clinical trial) were studied in a controlled randomized open prospective multicenter clinical trial in comparison with plasma derived FIX - Octanine® F (filtered) (Octapharmazeutika Produktionsges mbH, Austria). The main objective of this study was to test therapeutic equivalence and safety of nonacog alfa compared to Octanine F® in patients with severe and moderate hemophilia B. The safety and efficiency were studied in "on demand" treatment in 18 patients. According to randomization, patients were divided into 2 groups. Patients in the 1st group (n=9) received the nonacog alfa (Innonafactor) and patients of the 2nd group were given the Octanine F®. In the 1st group there were 6 patients with severe hemophilia B (activity of FIX was less than 1%) and 3 patients with moderate form of the disease (activity of FIX was 1-5%). In the 2nd group 4 patients had severe and 5 patients moderate hemophilia B. During the follow up period (13±1 week) 64 bleeding episodes were registered in patients of the 1st group; 51 of them (79.7%) were mild and 13 (20.3%) were moderate (Tab.1). In the 2nd group 70 bleeding episodes were recorded, of which 4 (5,7%) were mild, 65 (92,9%) were moderate and 1 (1.4%) was severe. The average number of bleeding episodes in patients of the 1st group (7,11±3,72) was slightly less than in patients of the 2nd group (7,78±5,22; p=0,93). The average total duration of bleeding, as well as the average duration of one bleeding episode were lower in patients of the 1st group (9,44±5,53 versus 13,78±15,39 days and 1,3±0,43 versus 1,65±1,38 days). The differences were statistically non-significant for both measures (p=0,86; p=0,93). The vast majority of bleedings were stopped with 1 - 2 doses of study drugs: 64 (100%) episodes in the 1st group and 67 (95,71%) episodes in the 2nd group (p=0,512). There were no difference in the average number of injections required to stop one bleeding episode 1,06±0,08 in the 1st group versus 1,39±0,89 in the second group (p=0,96).
During follow up period patients of the 1st group required fewer doses of drug to stop bleeding (7,56±3,91) than patients of the 2nd group (11,56±11,2), but the differences were statistically not significant (p=0,859). The average amount of drug that stanched one episode of bleeding was less in the 1st group (3346,01±801,51 ME) than in the 2nd one (4264,38±2987,58 ME; p=0,93). The average number of drug to stop all episodes of bleeding in one patient in the 1st group was less (23222,22±11659,52 ME) than in the 2nd group (35722,22±34726,71 ME; p=0,929). The average value of the maximum assessment of the pain severity by a visual analogue scale during episodes of bleeding in patients of the 1st group (5,5±1,6 points) did not differ significantly from the same indicator in patients of the 2nd group (5,38±score 1,19; p=0,914). The safety assessment was performed in 18 patients. There is no any side effects, infection transmission, or de novo inhibitor incident. Thus, the study showed that nonacog alfa is effective to stop bleeding in patients with severe and moderate hemophilia B, the results are comparable with the results of the use of Octanine F. Nonacog alfa is safe, and its use not accompanied with toxic, thrombogenic, immunogenic and allergic reactions.
Efficacy and Safety Evaluation
* p-values are presented descriptively (n=9).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.