Abstract
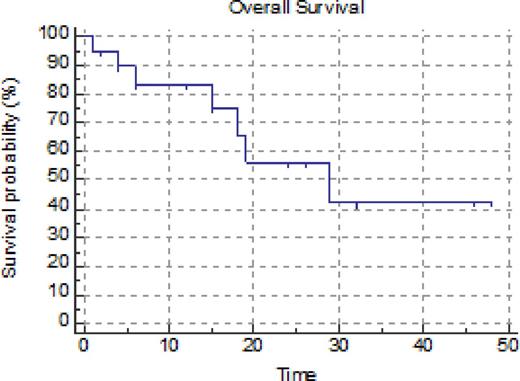
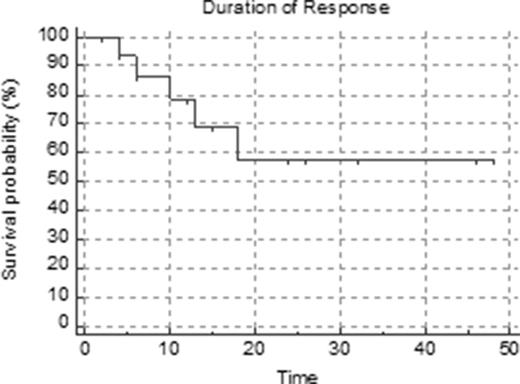
The CLAG regimen (G-CSF 300 mcg sc, cladribine 5 mg/m2 over 2 hours, and cytarabine 2 gm/m2 over 4 hours beginning 2 hours after cladribine, all daily times 5 days) was originally devised by Robak et al (Leuk Lymphoma 2000; 36:121-9) as induction therapy for patients with relapsed or refractory acute myeloid leukemia. Fifty percent of patients achieved a CR with a median duration of 22.5 weeks. This group subsequently added mitoxantrone to the regimen (Wrzesien-Kus A, et al. Ann Hematol 2005; 84:557-64). We treated 20 patients with previously untreated acute myeloid leukemia who were considered unsuitable for our intensive high-dose cytarabine, high-dose mitoxantrone frontline induction regimen either due to age or cardiac dysfunction with CLAG-based therapy. Patients with a cardiac ejection fraction above 50% additionally received either mitoxantrone (mito) or idarubicin (ida), 12 mg/m2 times 3 days, concurrently with CLAG. Of 20 patients treated, 5 received CLAG, 12 received CLAG-ida and 3 received CLAG-mito. The median age was 64 years (range 42-79 years). There were 13 men and 7 women. Six patients had received prior chemo and/or RT for a previous malignancy. In addition 3 patients had a prior MPD and 1 had prior MDS (total of 10 patients with secondary AML). Patients had a median of 3 comorbidities (range 0-7). Cytogenetic risk was good: 2 patients (however one was FLT3 ITD+), intermediate: 10 patients, poor: 8 patients. Only one patient was FLT3 ITD+. Responding patients (CR or PR) received 1 (5 pts), 2 (2 pts), 3 (8 pts) or 4 (2 pts) cycles of CLAG+/- ida or mito followed by allogeneic stem cell transplant (4 pts), hidac (2 pts) or decitabine maintenance (4 pts). Most patients responded to therapy. There were 13 formal CRs (65%), 1 CRp (5%), 3 PR (15%, defined as 6-10% blasts on marrow with complete hematologic recovery in the peripheral blood), 2 failures (10%) and one early death (5%). Other than the early death, treatment was well tolerated with few toxicities other than neutropenic fever and cytopenias. Estimated overall survival by Kaplan Meier analysis is 29.6 months (95% CI 20.1-39.2 months). Duration of response is 32.3 months (95% CI 21.6-43.1 months).
CLAG-based therapy is a well-tolerated, efficacious induction strategy in previously-untreated patients with high risk AML. CLAG-based regimens should be studied in a broader group of newly diagnosed AML patients.
Off Label Use: Use of cladribine in AML.
Author notes
Asterisk with author names denotes non-ASH members.