Abstract
Background: Relapsed or refractory acute lymphoblastic leukemia (ALL) remains challenging to treat with an extremely poor prognosis. Salvage chemotherapy regimens can achieve complete remission (CR) rates of 30-50%, but CRs are not durable without hematopoietic stem cell transplant (HSCT). Outcomes in untreated adolescents and young adults have improved with the use of pediatric chemotherapy regimens, but data on the use of pediatric chemotherapy regimens in relapsed/refractory adult ALL is lacking. We chose to retrospectively examine the outcomes of adult patients with relapsed ALL at our institution treated with the CCG-1941 pediatric salvage protocol (Gaynon PS, et al. J Clin Oncol 2006).
Methods: We conducted a single-center retrospective cohort study of patients aged 18 and older with relapsed/refractory ALL who were treated with the CCG-1941 protocol. Patients received induction with vincristine, dexamethasone, ifosfamide, etoposide, PEG-asparaginase, and methotrexate, as well as intrathecal methotrexate, cytarabine, and hydrocortisone prophylaxis, followed by intensification and continuation phases. This regimen was offered to relapsed/refractory patients who, in the judgement of treating physician, were likely to tolerate multiagent chemotherapy. All adult patients who received this regimen between 2006 and 2015 were included in the analysis. Outcomes of interest were: the CR rate, duration of remission (DOR), toxicity, 30-day mortality, and the rate of patients undergoing HSCT.
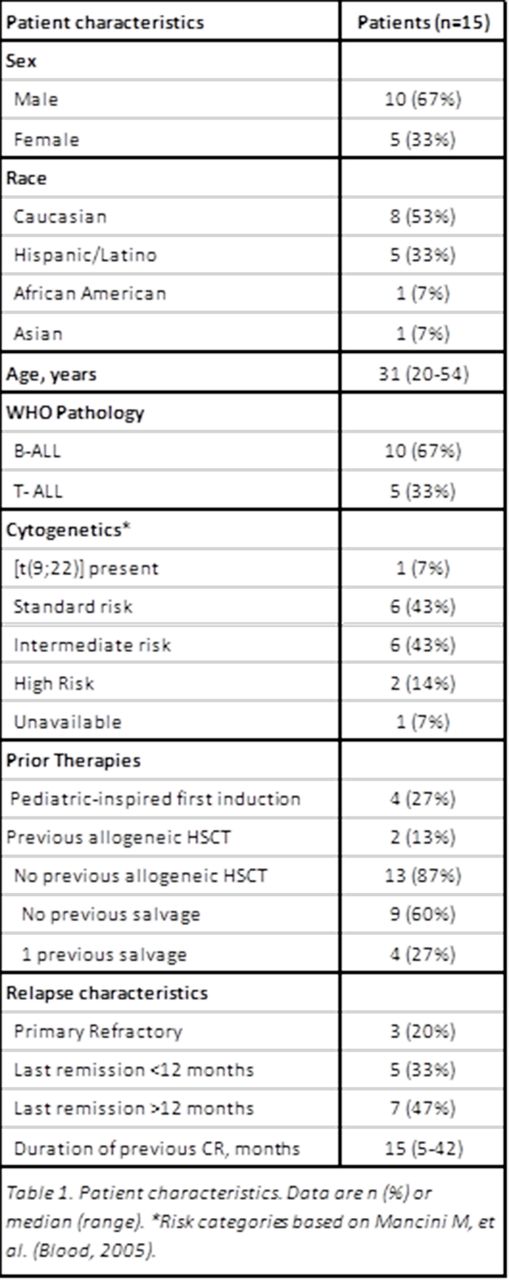
Results: Between January 2006 and April 2015, 15 patients aged 20-54 (median 31) with first relapse (n=12) or refractory (n=3) ALL were treated with the CCG-1941 regimen. Baseline patient characteristics are described in the Table 1. Seven patients (47%) had alterations to the induction protocol. The majority of these modifications were reduction or omission of PEG-asparaginase or vincristine. All patients experienced infectious complications, most commonly neutropenic fever (n= 12, 80%, 95% CI 52-96). There was one death due to infection, which occurred during an intensification phase. Two patients had grade 3 pancreatitis and two patients had hemorrhage (one grade 2, and one grade 5). 30-day mortality was 7% (95% CI 0-32) due to one fatal intracranial hemorrhage. Median length of hospitalization for induction was 28 days (range 10-61).
Twelve patients (80%, 95% CI 52-96) achieved CR, and six of these patients received 1-2 cycles of intensification or continuation. Among the remaining 6 CR patients, one proceeded immediately to HSCT, two received other consolidation, two had early relapse, and one was lost to follow up. Six patients proceeded directly to HSCT, and one more underwent HSCT after receiving subsequent therapies for relapsed disease. The DOR was 81% at 6 months, 54% at 12 months, 36% at 18+ months and 18% at 24 months. One patient has been followed for 63 months and has not recurred. Median follow-up for survivors was 16 months (range 3.6-63).
Conclusions: The CCG-1941 regimen appears to be tolerable and efficacious in adult patients with relapsed/refractory ALL. This regimen has been previously reported only in children. Despite the regimen's toxicities, a substantial proportion of patients underwent subsequent stem cell transplantation. Such salvage therapies remain important options for patients with T-ALL and for those B-ALL patients who are not candidates for, or who have failed phenotype-specific immunotherapies. Further prospective, multicenter study is warranted for use of pediatric salvage regimens in the adult patient population.
Foster:Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.