Abstract
Background:
The interaction of multiple myeloma (MM) cells with bone marrow (BM) cells along with factors in the BM milieu such as chemokines and cytokines play a crucial role in both progression of MM and drug resistance. Activation of the PI3-K/Akt survival pathway is a characteristic of both human MM cell lines and patient samples. This activation can be linked to BM microenvironmental signalling and use of proteasome inhibitors in treatment, suggesting this as a crucial point of therapeutic intervention to abrogate growth and survival signals in MM. However, the efficacy of such therapeutics has been modest and is likely to be compromised by the stimulation of compensatory signalling pathways, such as the PIM kinases, which like the PI3-K/Akt pathway are also induced by BM microenvironmental influences and share similar downstream targets. These proto-oncogenic kinases are constitutively active and play an important role in proliferation and survival in MM. The influence of these kinases on homing and migration has been observed in other malignancies, this has yet to be reported in MM. Here we report the effects of a dual inhibitor of PIM/PI3-K, IBL-202, and provide novel insights into effects on cell survival, signaling and migration.
Methods:
We investigated the effect of IBL-202 against a panel of MM cell lines (MM.IS, NCI-H929s, KMS11 and RPMI-8226) and primary MM patient samples. The in vitro efficacy of IBL-202 was compared to that of single pan-PIM inhibitors pPIMi and AZD1208 and also the pan-PI3-K inhibitor GDC-0941. Apoptosis was measured with AnnexinV staining and cell cycle analysed with Edu/DAPI staining.
To mimic BM microenvironmental conditions MM cells were cultured under hypoxic conditions (1% O2) and in co-culture with the human stromal cell line HS5.
Surface expression of CXCR4 was assessed in MM cell lines by flow cytometry. PIM kinases, pCXCR4 and downstream targets of PIM/PI3-K were examined by western blot. Transwell migration assays were carried out in the presence of 50ng SDF-1α for 4h @ 37o C.
Results:
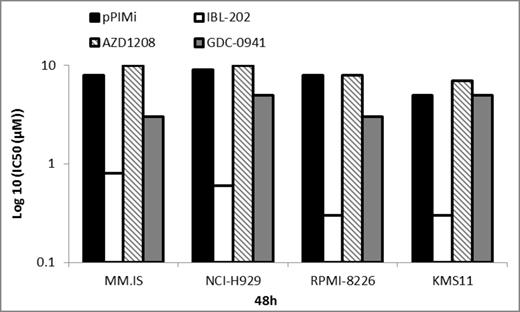
Simultaneous inhibition of PIM and PI3-K using IBL-202 in vitro was significantly more potent at inducing apoptosis than GDC-0941, pPIMi or AZD1208 in all MM cell lines tested. IC50 values were under 1μM for IBL-202 at 48h whilst in comparison the pan PIM inhibitors pPIMi and AZD1208 scored IC50 values between 5 and 10μM. The IC50 for GDC-0941 was on average 5μM (Figure 1).
At the molecular level there was a notable decrease in phosphorylation of known PIM/PI3-K targets Akt (Ser473), Bad (Ser112) and the translational targets S6 (Ser235/236) and 4EBP1 (Thr37/46). The levels of total proteins were unchanged.
Treatment with increasing doses of IBL-202 led to a marked reduction in cells in S phase of the cell cycle. These changes were paralled by down regulation of the cell cycle promoting proteins cyclin D1 and c-myc.
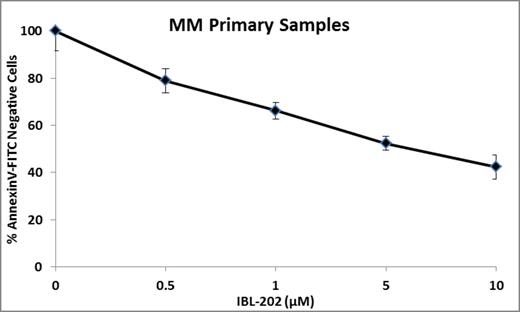
IBL-202 was also effective in inducing apoptosis in primary MM patient samples (n=4) after just 24h as assessed by Annexin-V staining (Figure 2).
To explore the role of the BM microenvironment we co-cultured MM cell lines with HS5s. This led to strong induction of PIM2 in MM cells. While MM cells in this setting were protected from Bortezomib-induced cell death, the apoptotic effect of IBL-202 was enhanced. In a further effort to mimic the tumour microenvironment we cultured MM cell lines in hypoxia. This may be of particular relevance as Pim-1 has been reported to be a pivotal regulator involved in hypoxia-induced chemoresistance. MM cells were further sensitised to IBL-202 in hypoxia. In addition, hypoxia increased the surface expression of CXCR4, a chemokine receptor critical for homing of MM cells to the bone marrow, with a concomitant increase in PIM1. Treatment of MM cell lines with IBL-202 reduced the level of PIM1 and CXCR4 Ser339 phosphorylation, along with down regulation of CXCR4 surface expression resulting in reduced migration of MM cells along an SDF-1 gradient.
Conclusion:
Together these data provide direct evidence of the potency of IBL-202 in MM in conditions that mimic the BM microenvironment. Moreover, they indicate a potential role for PIM kinases in facilitating dissemination and invasiveness of MM by CXCR4 and provide an added rationale for targeting PIM kinases in MM.
O'Neill:Inflection Biosciences: Employment.
Author notes
Asterisk with author names denotes non-ASH members.