Abstract
Background: The effects of spaceflight on human physiology are complex and represent a sum of many factors, the main two of which are microgravity and space radiation. The latter is composed of high-energy protons and HZE particles, along with secondary radiation produced in shielding and tissue. One major concern is short- and long-term radiation-induced injury to the hematopoietic system, one of the most radiosensitive tissues in the human body. Previous studies demonstrated functional and/or numerical changes in T and /or B-cells of crewmembers of STS-41B and STS-41D and altered myelopoiesis during STS-63 and STS-69. Although these changes reversed post return, they persisted in space and were more recently documented during a six month mission to the International Space Station. The main goal of NASA's space radiation research program is to enable human space exploration within acceptable risks and to reduce uncertainties regarding risk projections. We present the first ground based in vivo study evaluating the effects of high- and low-LET radiation on the human immune system by using a reconstituted human hematopoietic system in a humanized mouse model. We measure post-radiation depletion and repopulation kinetics, tumor formation and DNA damage.
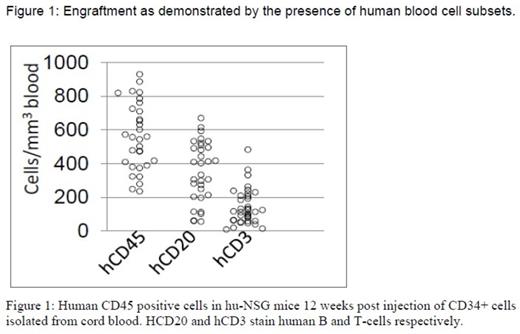
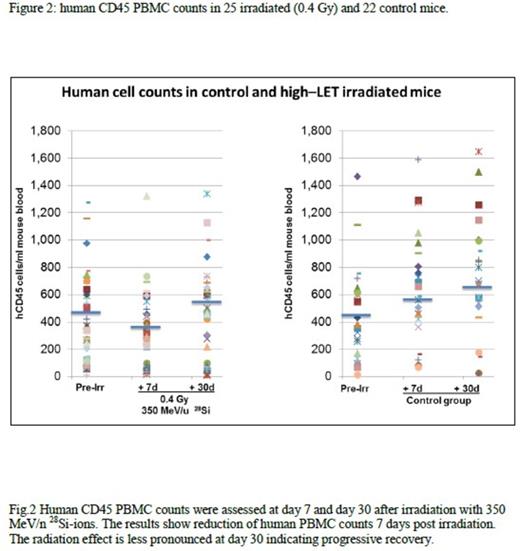
Methods: NSG mice were irradiated with 2Gy of gamma rays and injected 20 hours later with 2x105 CD34+cells isolated from cord blood. After a 12 week engraftment period, peripheral blood flow cytometry with anti-human and anti-mouse antibodies (human and mouse CD45, human CD3 and human CD19) was performed to confirm engraftment (>50% of human cells in moue blood, Figure 1). We irradiated the humanized-NSG mice (hu-NSG) with 0.2 or 0.4 Gy of 350 MeV/n28 Si-ions, which are established components of LET radiation. We measured changes in peripheral blood mononuclear cell counts (huCD45+PBMC) 7 and 30 days post irradiation to assess early effects and recovery kinetics. Multicolor flow cytometric immunophenotyping (FCI) analysis of tissue and blood samples was performed according to standard cell surface staining protocols. Conventional cytogenetic analysis was performed 5 months post radiation on enriched fractions of human HSC and progenitor cells, isolated from marrow of irradiated mice. We performed secondary transplants to transmit the genetic changes imparted by irradiation in the first animal to the secondary host system to allow further monitoring. In addition whole exome and transcriptome sequencing is underway on stem cell and progenitor (lineage negative) pools on a subset of mice.
Results: To date 120 well engrafted NSG mice were irradiated with 0.2 or 0.4 Gy of 350 MeV/n28 Si-ions. No decrease versus a mild decrease of huCD45+ PBMCs was observed on day 7 in the 0.2 and 0.4 Gy radiated cohort respectively, with the latter demonstrating full recovery on day 30 compared to normal control mice (Figure 2). No lymphoproliferative or myeloproliferative disorder was noted in 46 irradiated mice using 8 color FCI analyses. Median percentage of peripheral blood mature B-cells, T cells and NK cells was 41%, 36.5% and 9% respectively. Kappa /lambda ratio, CD4/CD8 ratio and NK cell/T cell ratio were 0.9, 3.6, and 0.3 respectively. Bone marrow evaluation demonstrated 0.08% to 0.3% myeloblasts (median: 0.15%) positive for CD34 /CD117/CD33 and HLADR. Moderate monocytosis (CD64+/CD4+/HLA-DR+) was noted, ranging from 39% to 51% (median 47%). Preliminary results of cytogenetic analysis revealed diploid male and female karyotypes without abnormalities in at least 20 metaphases analyzed. Successful secondary transplants were performed in 30 mice. Results of whole exome and transcriptome sequencing are pending.
Conclusions: This study shows for first time data on the effects of high -LET space type of radiation on human hematopoietic system in vivo. Preliminary results show that high-LET 350 MeV/n 28 Si radiation has relatively minor effects on peripheral blood cell counts and depletion/recovery kinetics. In addition our preliminary analysis did not demonstrate a leukemogenic effect in the short time period (months) assessed after the irradiation. The study is currently in progress and more data will be available.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.