Abstract
Introduction: Acute myeloid leukemia (AML) is a heterogeneous clonal disorder of the hematopoietic progenitor cells characterized by excessive proliferation of early progenitor cells in the bone marrow (BM) and is hence associated with a variable prognosis. BM niches are specialized types of microenvironment that actively support the growth, maturation and maintenance of hematopoietic stem and progenitor cells. They include a complex network of extracellular matrix proteins, soluble growth factors, and cytokines. Little is known about the leukemic microenvironment or its clinical relevance to AML initiation and propagation. The aim of the study was to define the role of stromal cells in support of leukemic cell growth in active disease as compared to the remission (Rm) state and normal stoma. Thus, we have investigated the expression of various cytokines secreted by stromal cells and involved in hematopoietic progenitor maintenance and proliferation at disease diagnosis (Dx) and Rm. Additionally, we have characterized the growth pattern of leukemic cells on various stroma cells from different sources and disease stages.
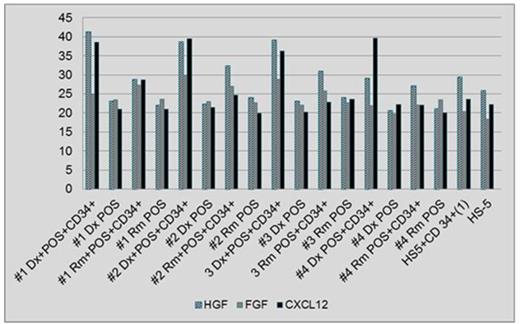
Methods: Leukemic and stromal cells from BM of 4 patients with AML were collected at Dx. Stromal cells were also collected in Rm. Leukemic cells were enriched for CD34+ using magnetic beads. Patients' own stroma (POS) obtained both at Dx and Rm and the HS-5 cell line (control) were seeded on gelatin-treated plates and were grown with MesenCult medium for 42 days. Morphologic and growth rate changes were monitored every week using inverted microscopy X20. Upon adherence of 40-50% of the stromal cells, CD34+ enriched leukemic cells derived at Dx were seeded on top of POS from both Dx and Rm and were grown together for additional 2 weeks. Co-cultures were harvested and RNA was extracted followed by RT-PCR to amplify the following hematopoietic regulating genes: hepatocyte growth factor (HGF), fibroblast growth factor (FGF) and CXCL12. The results were expressed as arbitrary units normalized to GAPDH gene expression.
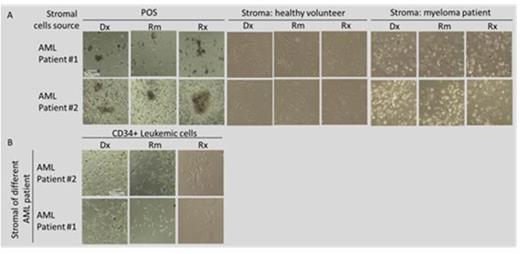
Results: Expression of CXCL12 and HGF was elevated in a co-culture of leukemic cells and POS obtained at Dx compared to POS from Rm in all 4 patients. Additionally, the co-culture of leukemic cells and POS from Rm showed a higher expression of CXCL12 and HGF as compared to POS alone derived in Rm (Fig. 1). Mean values of cytokine expression were 37.33, 26.44 and 38.62 at Dx and 29.83, 25.55 and 24.55 in Rm for HGF, FGF and CXCL12, respectively. Additionally, a preferential growth advantage of colonies derived from single leukemic cells was observed when these cells were seeded on POS from Dx and relapse (Rx) compared to Rm (Fig. 2A). Moreover, colonies grew mainly in a co-culture with POS while no growth was noted on healthy stromal cells or stroma derived from a patient with myeloma (Fig. 2A) or another AML patient (Fig. 2B).
Conclusions: Co-culture of AML cells and POS derived at Dx show a higher expression of CXCL12 and HGF compared to POS obtained in Rm, suggesting favorable microenvironment conditions supporting AML initiation and maintenance. A synergistic effect on expression of CXCL12 and HGF was observed in a co-culture of POS with leukemic cells as compared to POS or cell-line alone. Leukemic cells show preferential growth on POS and no growth on various malignant and normal stromal cells. Leukemic cell growth was diminished in Rm compared to Dx and Rx. Studying a larger number of patients as well as investigating the specific interaction of AML cells with stromal cells derived from various disease stages is needed to confirm the above results.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.