Abstract
Introduction
Antithymocyte globulin (ATG) is commonly used for graft-versus-host disease prophylaxis in allogeneic hematopoietic stem cell transplantation. However side effects of ATG is frequent and is caused by the cross reaction with lymphocytes and macrophages. Despite premedication with corticosteroids, acetaminophen and antihistamine drugs; complications like fever, chills, skin rashes, anaphylactic shock, nephritis and serum sickness occur frequently during or after ATG infusion. Hence, we have recently decided to use higher doses of methylprednisolone as premedication, with the aim of reducing frequency and severity of acute -and late- complications related to ATG administration. In this retrospective study we compared two different premedication protocols in terms of adverse reactions, clinical and laboratory changes that was observed during/after ATG infusion.
Patients and methods
28 patients were included in this study. Patients were divided into two groups according to premedication protocol (Table 1). All patients received ATG-Fresenius (ATG-F; Fresenius Biotech GmbH, Munich, Germany) over 10 hours for 3 days. As clinical parameters fever, heart rate, blood pressure, weight gain, existence of skin rashes, chills, dyspnea, thrombocyte transfusion requirement and diuretic use were recorded from the first day to fourth day of ATG-F infusion. As laboratory parameters leucocyte and thrombocyte counts, glucose, blood urea nitrogen, creatinine, alanine aminotransferase, aspartate aminotransferase, total bilirubin, lactate dehydrogenase (LDH), C-reactive protein (CRP), procalcitonin, fibrinogen, D-dimer, prothrombin time and activated partial thromboplastin time values were evaluated from the first day to fourth day of ATG-F infusion. Day 1 values were obtained before ATG infusion and day 4 values were obtained after the day that ATG infusion finished. Lymphocyte counts at the beginning of conditioning regimen and before first ATG-F infusion day were also recorded. Mann-Whitney U and chi-square test were used for comparisons between two groups.
Premedication protocols
| Protocol A . | 1 mg/kg methylprednisolone, 45 mg pheniramine and 1000 mg acetaminophen one hour before ATG-F. 45 mg pheniramine and 1000 mg acetaminophen were repeated at middle of ATG-F infusion . |
|---|---|
| Protocol B | 1 mg/kg methylprednisolone, 45 mg pheniramine 12 hours before ATG-F infusion 3 mg/kg methylprednisolone, 45 mg pheniramine 12 hours before ATG-F infusion 1 mg/kg methylprednisolone, 45 mg pheniramine at middle of ATG-F infusion |
| Protocol A . | 1 mg/kg methylprednisolone, 45 mg pheniramine and 1000 mg acetaminophen one hour before ATG-F. 45 mg pheniramine and 1000 mg acetaminophen were repeated at middle of ATG-F infusion . |
|---|---|
| Protocol B | 1 mg/kg methylprednisolone, 45 mg pheniramine 12 hours before ATG-F infusion 3 mg/kg methylprednisolone, 45 mg pheniramine 12 hours before ATG-F infusion 1 mg/kg methylprednisolone, 45 mg pheniramine at middle of ATG-F infusion |
Results
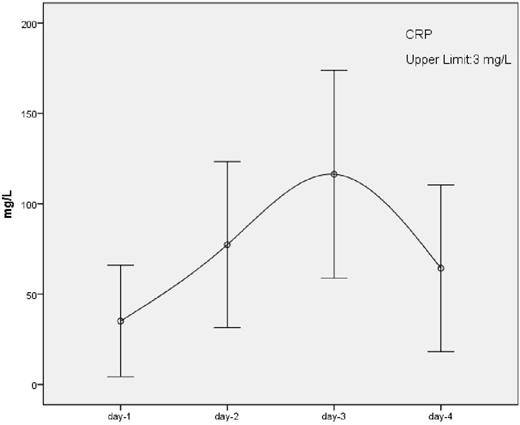
Patients' characteristics of two groups are shown in table 2. Median age, patient number, gender and underlying disease distribution was similar for both groups. There was no significant difference between two groups according to laboratory parameters. For the clinical parameters, only day 2 "weight gain" was significantly higher in protocol B group and remaining parameters were insignificant. Frequency of hyperglycemia was similar. Regardless of the premedication protocol, D-dimer and CRP values increased to very high levels toward day 3 and then started to decrease (Figure 1). D-dimer and LDH levels at day 2 (p<0.05, p<0.001), day 3 (p<0.05, p<0.001) and day 4 (p<0.001, p<0.001) were positively correlated with pre-ATG lymphocyte count at the first day.
Patients' characteristics
| . | Protocol A . | P value . | Protocol B . |
|---|---|---|---|
| n | 13 | 15 | |
| Age (median) | 27 | NS | 38 |
| Sex (M/F) | 6/7 | NS | 7/8 |
| Underlying disease Acute myeloid leukemia Acute lymphoblastic leukemia Chronic myeloid leukemia Fanconi aplastic anemia Aplastic anemia Myelodysplastic syndrome Hodgkin lymphoma Myelofibrosis | 5 3 2 2 0 1 0 0 | NS | 4 3 1 0 3 1 2 1 |
| Conditioning regimen Myeloablative Reduced intensity | 7 6 | NS | 12 3 |
| Disease status First remission Second remission Primary refractory Partial response | 4 2 4 0 | NS | 4 1 4 2 |
| Total ATG-F dose 30 mg/kg 60 mg/kg 90 mg/kg | 5 6 2 | NS | 5 8 2 |
| . | Protocol A . | P value . | Protocol B . |
|---|---|---|---|
| n | 13 | 15 | |
| Age (median) | 27 | NS | 38 |
| Sex (M/F) | 6/7 | NS | 7/8 |
| Underlying disease Acute myeloid leukemia Acute lymphoblastic leukemia Chronic myeloid leukemia Fanconi aplastic anemia Aplastic anemia Myelodysplastic syndrome Hodgkin lymphoma Myelofibrosis | 5 3 2 2 0 1 0 0 | NS | 4 3 1 0 3 1 2 1 |
| Conditioning regimen Myeloablative Reduced intensity | 7 6 | NS | 12 3 |
| Disease status First remission Second remission Primary refractory Partial response | 4 2 4 0 | NS | 4 1 4 2 |
| Total ATG-F dose 30 mg/kg 60 mg/kg 90 mg/kg | 5 6 2 | NS | 5 8 2 |
NS: Not significant
Discussion
Our study showed that high dose corticosteroid use for ATG-F premedication is not effective in prevention of adverse events. ATG caused D-dimer, CRP and LDH elevations that could be related to cytokine release and this effect was positively correlated with pre-ATG lymphocyte number. Probably lysis of lymphocytes and destruction of T regulatory cells by ATG may initiate and enhance this reaction. This correlation may help to predict adverse reactions due to ATG.
CRP: C-reactive protein
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.