Abstract
Chronic extensive, severe graft-versus-host-disease (GVHD) is a major cause of morbidity and mortality following an allogeneic hematopoietic stem cell transplant (HSCT). Patients with progressive chronic GVHD (cGVHD) following standard treatments have limited options. Patients with extensive refractory cGVHD have a decreased performance status (PS) and an increased non-relapsed mortality. Extensive sclerodermatous cGVHD responds poorly to standard treatments. Colchicine is a well-known anti-inflammatory drug that has been used in the treatment of many inflammatory disorders (1). We report a series of 8 patients with extensive cGVHD who were refractory to multiple lines of therapy and then were treated with colchicine. All patients had improvements in ECOG PS, skin GVHD and/or oral GVHD after starting colchicine. All patients were able to decrease their immunosuppressive therapies (IST) after starting colchicine.
We retrospectively examined 8 patients with refractory extensive cGVHD, treated with colchicine (0.6 mg QD or BID). IST for cGVHD was defined by the following: steroids, extracorporeal photopheresis (ECP), imatinib, mycophenolate mofetil (MMF), tacrolimus, bortezomib, montelukast, cyclophosphamide (Cy), pentostatin, and rituximab. National Institutes of Health 2014 cGVHD consensus criteria (2) were used to grade the cGVHD prior to colchicine and at the time of best response after colchicine was started.
Baseline patient characteristics are listed in Table 1. Conditioning regimen for allogeneic HSCT consisted of PPT (ECP, pentostatin, TBI) (4/8), busulfan/Cy (2/8), Cy-TLI (1/8), or Cy/TBI (1/8). GVHD prophylaxis involved CSA/MTX (7/8), tacrolimus/MTX (1/8) and ATG in addition to CSA/MTX (1/8). Median number of organs involved in cGVHD was 4.5 (range 2-5). All patients had extensive cGVHD with skin (8/8), oral (8/8), eye (8/8), GI (7/8), lung (6/8), liver (2/8), and/or genital (2/8) involvement. Median number of failed IST was 3.5 (range 1-6). Patients failed steroids (7/8), ECP (6/8), MMF (5/8), imatinib (3/8), cyclophosphamide (2/8) and bortezomib (1/8). Median number of IST immediately before colchicine was 2.5 (range 1-6). Most patients required twice daily dosing of colchicine (5/8). Median follow-up since beginning colchicine was 11 weeks (range 3-67 weeks).
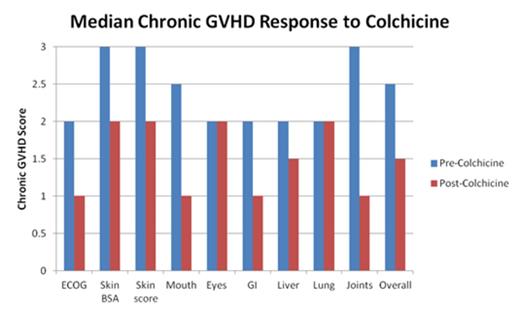
At best response with colchicine, all patients had clinical improvement of chronic GVHD (Figure 1). Patients with extensive cGVHD of the skin, including all patients with extensive sclerodermatous changes, responded to the addition of colchicine, and had steroid and MMF doses lowered, and/or were stopped on IST. Median number of IST fell to 2 (range 0-5). Four patients had a reduction in the number of IST. One patient was taken off 4 IST (ECP, imatinib, Cy and MMF). Another patient was able to stop ECP. Five patients had a reduction in prednisone with median reduction of 5mg (range 5-40 mg), while 2 had reductions in the total daily dose of MMF. Adverse events were uncommon and included 2 patients with grade 1-2 diarrhea. The most severe adverse event was grade 2 diarrhea in one patient that led to eventual discontinuation of colchicine. No hematologic toxicity was observed. The rest (7/8) continued on colchicine therapy.
In conclusion, patients with extensive cGVHD involving the skin and mouth, refractory to multiple lines of IST, experienced clinical improvement on colchicine. Toxicity was limited to mild GI side effects. These observations need to be validated in a prospective clinical trial.
References
(1) Slobodnick et al. Am J Med. 2015 May;128(5):461-70.
(2) Jagasia et al. Biol Blood Marrow Transplant. 2015 Mar;21(3):389-401.e1. Epub 2014 Dec 18.
Baseline Characteristics
| Variable . | Frequency (%) . |
|---|---|
| Age, median (range), yr | 57 (30-65) |
| Donor/recipient gender | |
| Sex-mismatched female/male | 1 (13%) |
| Sex-matched | 7 (88%) |
| Race | |
| White/Caucasian | 7 (88%) |
| Disease category | |
| Acute myeloid leukemia | 4 (50%) |
| Myelofibrosis | 2 (25%) |
| Aplastic anemia | 1 (13%) |
| Lymphoblastic lymphoma | 1 (13%) |
| Remission status at transplantation | |
| CR | 8 (100%) |
| Graft source | |
| BM | 1 (13%) |
| PBSC | 7 (88%) |
| Donor type | |
| Matched sibling | 4 (50%) |
| Matched unrelated | 3 (38%) |
| Mismatched | 1 (13%) |
| GVHD type | |
| Progressive | 3 (38%) |
| Overlap syndrome | 2 (25%) |
| De novo | 3 (38%) |
| Variable . | Frequency (%) . |
|---|---|
| Age, median (range), yr | 57 (30-65) |
| Donor/recipient gender | |
| Sex-mismatched female/male | 1 (13%) |
| Sex-matched | 7 (88%) |
| Race | |
| White/Caucasian | 7 (88%) |
| Disease category | |
| Acute myeloid leukemia | 4 (50%) |
| Myelofibrosis | 2 (25%) |
| Aplastic anemia | 1 (13%) |
| Lymphoblastic lymphoma | 1 (13%) |
| Remission status at transplantation | |
| CR | 8 (100%) |
| Graft source | |
| BM | 1 (13%) |
| PBSC | 7 (88%) |
| Donor type | |
| Matched sibling | 4 (50%) |
| Matched unrelated | 3 (38%) |
| Mismatched | 1 (13%) |
| GVHD type | |
| Progressive | 3 (38%) |
| Overlap syndrome | 2 (25%) |
| De novo | 3 (38%) |
Off Label Use: Colchicine will be discussed as treatment for GVHD. Comenzo:Karyopharm: Research Funding; Prothena: Research Funding; Takeda Millennium: Research Funding; Janssen: Research Funding; Takeda Millennium: Membership on an entity's Board of Directors or advisory committees; Prothena: Membership on an entity's Board of Directors or advisory committees. Roberts:Millenium: Speakers Bureau. Miller:Biogen Idec: Consultancy; AbbVie: Speakers Bureau; Millennium: Speakers Bureau; Onynx: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.