Abstract
Background: Retrospective studies have shown that achieving minimal residual disease (MRD) by multi-parameter flow cytometry (MFC) is predictive of outcomes in multiple myeloma (MM). Modern induction therapies including bortezomib (V), lenalidomide (R) followed by autologous hematopoietic cell transplant (AHCT) have resulted in substantial improvements in progression free survival (PFS) and overall survival (OS). The impact of bortezomib-based therapy on end of induction chemotherapy MRD status and post-AHCT MRD status is not well defined. We designed a prospective clinical trial (NCT01215344) to study the incidence of MRD negativity at end of induction and following AHCT, and its association with PFS and OS.
Patients and Methods: Twenty patients with newly diagnosed, symptomatic MM were enrolled. They received four cycles of VRd induction therapy followed by AHCT. Dose modifications of VRd were controlled during the clinical trial. MRD status by MFC was evaluated at the end of induction chemotherapy and at day 100 post-AHCT. The MFC cut-off to determine MRD negativity was defined as 10-4. Outcomes analyzed included MM disease status, PFS and OS.
Results: Three cohorts of patients were identified; patients achieving MRD negative status at the end of induction (cohort 1, 50%), those achieving MRD negative status only after AHCT (cohort 2, 15%) and patients never achieving MRD negative status (cohort 3, 35%). All patients achieving MRD negative status at the end of induction remained MRD negative post-AHCT. There were no significant differences in median age, renal function, disease stage, cytogenetic risk and maintenance chemotherapy post-AHCT between cohorts. The table summarizes characteristics of these cohorts.
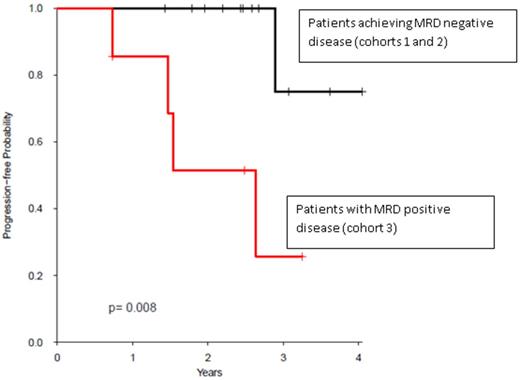
Patients who never achieved MRD negative status after AHCT had significantly shorter PFS (cohort 3) compared with patients who achieved MRD negative status (cohorts 1 and 2, p=0.008) (figure). The median PFS for cohort 3 was 2.64 years and not yet reached for the other cohorts. There were no significant differences in OS. Median follow-up for survivors was 2.53 years (range, 0.73-4.04).
Conclusions: In this study, bortezomib-based therapy resulted in half of patients achieving MRD negative status with induction chemotherapy alone. AHCT improved the depth of response with 30% of patients who were MRD positive after induction therapy converting to MRD negativity following AHCT. Achieving a negative MRD state, pre- and at D100 post-AHCT resulted in improved PFS. These findings were independent of molecular cytogenetics or ISS stage. MRD positive status at day 100 post-AHCT is highly predictive of earlier disease progression, and may help identify patients for alternative management approaches. Delay of AHCT may be considered as a potential management option for patients with MRD negative status at the end of induction therapy, as being studied in the IFM DFCI study (NCT01208662). As some patients with MRD positive disease at the end of induction therapy become MRD negative with AHCT, these patients may benefit from AHCT and this population warrants further investigation.
Descriptive analysis
| . | Cohort 1 MRD -/- (%) (n=10) . | Cohort 2 MRD +/- (%) (n=3) . | Cohort 3 MRD +/+ (%) (n=7) . | Test Statistic . |
|---|---|---|---|---|
| Median Age | 55 | 54 | 60 | 0.31 |
| ISS Stage III | 40 | 33 | 14 | 0.52 |
| DS Stage III | 89 | 67 | 60 | 0.14 |
| Median Serum creatinine | 0.90 | 0.60 | 0.88 | 0.10 |
| Serum M-spike | 2.0 | 1.9 | 2.1 | 0.80 |
| Bone marrow plasma cells | 14 | 44 | 14 | 0.18 |
| Cytogenetics | 0.37 | |||
| Standard risk | 40 | 0 | 57 | |
| Intermediate risk | 50 | 67 | 43 | |
| High risk | 10 | 33 | 0 |
| . | Cohort 1 MRD -/- (%) (n=10) . | Cohort 2 MRD +/- (%) (n=3) . | Cohort 3 MRD +/+ (%) (n=7) . | Test Statistic . |
|---|---|---|---|---|
| Median Age | 55 | 54 | 60 | 0.31 |
| ISS Stage III | 40 | 33 | 14 | 0.52 |
| DS Stage III | 89 | 67 | 60 | 0.14 |
| Median Serum creatinine | 0.90 | 0.60 | 0.88 | 0.10 |
| Serum M-spike | 2.0 | 1.9 | 2.1 | 0.80 |
| Bone marrow plasma cells | 14 | 44 | 14 | 0.18 |
| Cytogenetics | 0.37 | |||
| Standard risk | 40 | 0 | 57 | |
| Intermediate risk | 50 | 67 | 43 | |
| High risk | 10 | 33 | 0 |
Kaplan-Meier curve of progression-free survival comparing patients achieving MRD negative disease with MRD positive disease.
Kaplan-Meier curve of progression-free survival comparing patients achieving MRD negative disease with MRD positive disease.
Cornell:Prothena: Research Funding. Jagasia:Takeda Inc: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.