Abstract
BACKGROUND: In chronic phase chronic myeloid leukemia (CP-CML) patients treated with frontline imatinib, failure to achieve early molecular response (EMR failure: BCR-ABL1 >10% at 3 months) predicts for subsequent inferior outcomes. Identifying patients at high-risk of EMR failure provides an opportunity to improve outcomes by personalising treatment at the time of diagnosis, as intervention after EMR failure may be less effective.
AIM: To utilise a predictive gene signature to identify CP-CML patients at diagnosis, who are at high risk of EMR failure and inferior clinical outcomes.
METHODS: Peripheral blood mononuclear cells collected from 119 patients enrolled in the TIDEL-II study were subjected to gene expression microarray profiling (GEP) Illumina HT12. Validations of the identified microarray genes were performed using Taqman qPCR. All patients commenced imatinib treatment, and switched to nilotinib with or without an antecedent trial of high dose imatinib if they failed to achieve time dependent molecular targets. Clinical outcomes included EMR and cumulative incidence of MMR and MR4.5 (BCR-ABL1 ≤0.1% and ≤0.0032% on the international scale, respectively), and comparisons were made using Fine and Gray test. Competing risks included permanent trial discontinuation for any reason (including death or progression). Event-free survival (EFS) and failure-free survival (FFS) were performed using Kaplan-Meier and comparisons were made using the log-rank test.
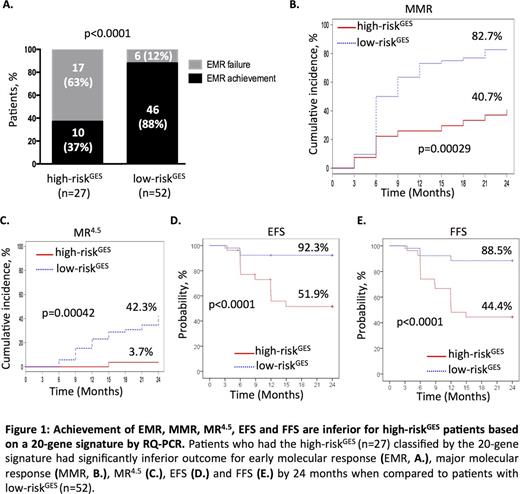
RESULTS: Fourteen of the 119 patients demonstrated EMR failure (12%). Comparing the GEP of these patients with those that achieved EMR identified 4456 aberrantly expressed genes in the EMR failure group. This gene set was significantly enriched for stem cell phenotype/signalling (e.g. Myc, β-catenin, Hoxa9/Meis1), cell cycle, and reduced immune response pathways associated with adverse prognosis in other cancers. From these genes, 20 genes (IGFBP2, CD3E, RASGRP1, BNIP3L, ETS1, PDK1, METTL7A, HECA, COL8A2, PRSS57, TMEM167A, SPAST, FZD7, VPS41, CDKN1B, CPXM1, SEPT7, RPS28, SLX4IP, and SRSF11) validated by qPCR were selected by nearest shrunken centroid model as the high-risk gene expression signature (high-riskGES) to predict EMR failure. Patients who had a high-riskGES exhibited significantly higher rates of EMR failure compared to those with low-riskGES (training cohort: 73.3% vs 8.0%; p<0.0001; n=40, Hazard Ratio (HR): 4.1). This was validated on an independent patient cohort (validation cohort: 50.0% vs 14.8%; p=0.018; n=39; HR: 3.2). Overall, when both cohorts were combined, patients who had a high-riskGES exhibited significantly higher rates of EMR failure compared to those with low-riskGES (63.0% vs 11.5%; p<0.0001; n=79, HR: 3.3; Figure 1A). The overall prediction accuracy of the signature was 80% (82% specificity, 74% sensitivity). Additionally, patients with a high-riskGES demonstrated significantly worse clinical outcome than those with low-riskGES by 24 months (MMR: 41% vs 83%, p=0.0003; MR4.5: 4% vs 42%, p=0.0004; EFS: 52% vs 92%, p<0.0001; FFS: 44% vs 89%, p<0.0001) (Figure 1B-E).
This high-riskGES was confirmed as an independent predictor for EMR failure, when Sokal, age and gender were added as covariates based on the Cox-proportional multivariate analysis (HR: 0.34, p=0.003). Patients who had a high-riskGES also had significant inferior outcomes even if they subsequently achieved EMR, compared to the low-riskGES patient group that subsequently achieved EMR (MR4.5: 10% vs 48%, p=0.034; EFS: 68% vs 96%, p=0.0099; FFS: 60% vs 91%, p=0.011). Furthermore, this 20-gene signature compared favourably to Sokal, EUTOS, Hasford, and OCT-1 Activity in predicting EMR failure based on assessing their respective overall performance F -score (harmonic mean of precision and sensitivity). EMR failure was observed in 15% (n=33) of low Sokal score patients overall and 12% of the low-riskGES group (n=49) but amongst patients who had both low-riskGES and a low Sokal score, 0/25 experienced EMR failure.
SUMMARY: For the first time in the CML setting, we have identified and validated a 20-gene signature to predict, at the time of diagnosis, patients at high risk of EMR failure and subsequent inferior clinical outcomes. The ability to predict high risk patients at diagnosis may facilitate the assessment of novel therapeutic approaches designed to improve clinical outcomes for patients with aggressive disease.
Yeung:BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Ariad: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. White:Novartis: Honoraria, Research Funding; BMS: Honoraria, Research Funding. Hughes:ARIAD: Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria, Research Funding; Novartis: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract