To the editor:
Monoclonal antibodies (mAbs), such as the anti-CD20 mAb rituximab, have transformed the treatment of malignant disease and are now a first-line treatment of many hematologic conditions. Although in many cases their precise mechanism of action is not fully elucidated, where target cell deletion is critical to effective treatment, Fcγ receptors (FcγRs) are now accepted to be directly involved.1-3 There are 4 FcγRs in mice and 6 in humans4 ; debate still exists over which FcγRs play the dominant role(s).
Early work investigating this used FcγRI- or FcγRIII-deficient mice and/or FcγRIV blockade using the hamster antibody 9E9,5,6 highlighting a critical role for FcγRI and FcγRIV while indicating redundancy for FcγRIII.7
Here, we investigated the role of different activatory FcγRs in the context of anti-CD20 mAb-mediated B-cell depletion. Using adoptive transfer experiments in FcγR-deficient mice, we unexpectedly showed that, in addition to blocking FcγRIV, 9E9 also binds and blocks FcγRIII in vivo. This blocking occurs via the 9E9 Fc, and only occurs when 9E9 first binds FcγRIV on the same effector cell, resulting in concurrent inhibition of FcγRIII and FcγRIV, blunting target deletion. Importantly, this activity was not detected with isotype controls and highlights an important paradigm in FcγR blocking, which may contribute to different interpretations of which FcγRs are critical for in vivo function.
Using adoptive transfer assays, we assessed the ability of Ritm2a8 to deplete human CD20 (hCD20) transgenic (Tg) B cells (Figure 1A). In line with previous findings,5-7 deficiency of a single activatory receptor did not affect depletion. We next explored the contribution of multiple activatory FcγRs, using a combination of FcγR−/− mice and 9E9 (Figure 1B). Blocking FcγRIV significantly reduced B-cell depletion and, in the absence of FcγRI, activity was lost completely, supporting that FcγRI and FcγRIV are the key FcγRs mediating depletion, in agreement with other studies.5-7,9
9E9 binds to and blocks both FcγRIII and FcγRIV in vivo. (A) Adoptive transfer studies in WT or FcγR−/− mice. Mice were injected IV with a 1:1 mix of target hCD20 Tg (T) and WT nontarget (nT) splenocytes differentially stained with CFSE (50 and 5 µM, respectively) as previously described.8 Approximately 20 hours later, mice were injected with irrelevant mAbs or Ritm2a (10 µg) and then 24 hours later spleens were stained with anti-mouse CD19 (1D3) before assessment by flow cytometry and the T:nT ratio of B cells in the spleen calculated. (B) Adoptive transfer studies comparing FcγRI−/− and FcγRIII−/− mice in combination with 9E9. Mice were treated with 9E9 (400 µg) 3 to 4 hours before injection of Ritm2a (10 µg) as in panel A. (C) SPR data demonstrating the binding affinity of 9E9 or de-gly9E9 to (i) FcγRI, (ii) FcγRIIb, (iii) FcγRIII, and (iv) FcγRIV. Anti-His antibody (R&D Systems) was bound to a CM5 sensor chip and His-tagged mFcγR (R&D Systems) captured. 9E9 or de-gly9E9 antibodies were then injected (200 nM) at 30 µL per minute. Association was monitored for 5 minutes and dissociation monitored for 10 minutes. (D) Adoptive transfer studies using de-gly9E9 in either FcγRI−/− or FcγRIII−/− mice. To produce de-gly9E9, 9E9 was treated with 0.05 U of PNGase F per µg at 37°C overnight. Deglycosylation was confirmed by SDS-PAGE and/or SPR. Purification of antibody from enzyme was achieved through size-exclusion chromatography using Sephadex 200. Adoptive transfer assay was carried out as in panel B; however, mice were treated with de-gly9E9 (400 µg) 3 to 4 hours before injection of Ritm2a (N = 4). Statistical analysis was performed using 2-way ANOVA. Experiments were cleared through local ethical committees and performed under home office license PPL30/2964. ANOVA, analysis of variance; CFSE, carboxyfluorescein succinimidyl ester; RU, resonance units; SDS-PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis. ***P = .0003; ****P < .0001 (2-way ANOVA, multiple comparisons using Tukey's test).
9E9 binds to and blocks both FcγRIII and FcγRIV in vivo. (A) Adoptive transfer studies in WT or FcγR−/− mice. Mice were injected IV with a 1:1 mix of target hCD20 Tg (T) and WT nontarget (nT) splenocytes differentially stained with CFSE (50 and 5 µM, respectively) as previously described.8 Approximately 20 hours later, mice were injected with irrelevant mAbs or Ritm2a (10 µg) and then 24 hours later spleens were stained with anti-mouse CD19 (1D3) before assessment by flow cytometry and the T:nT ratio of B cells in the spleen calculated. (B) Adoptive transfer studies comparing FcγRI−/− and FcγRIII−/− mice in combination with 9E9. Mice were treated with 9E9 (400 µg) 3 to 4 hours before injection of Ritm2a (10 µg) as in panel A. (C) SPR data demonstrating the binding affinity of 9E9 or de-gly9E9 to (i) FcγRI, (ii) FcγRIIb, (iii) FcγRIII, and (iv) FcγRIV. Anti-His antibody (R&D Systems) was bound to a CM5 sensor chip and His-tagged mFcγR (R&D Systems) captured. 9E9 or de-gly9E9 antibodies were then injected (200 nM) at 30 µL per minute. Association was monitored for 5 minutes and dissociation monitored for 10 minutes. (D) Adoptive transfer studies using de-gly9E9 in either FcγRI−/− or FcγRIII−/− mice. To produce de-gly9E9, 9E9 was treated with 0.05 U of PNGase F per µg at 37°C overnight. Deglycosylation was confirmed by SDS-PAGE and/or SPR. Purification of antibody from enzyme was achieved through size-exclusion chromatography using Sephadex 200. Adoptive transfer assay was carried out as in panel B; however, mice were treated with de-gly9E9 (400 µg) 3 to 4 hours before injection of Ritm2a (N = 4). Statistical analysis was performed using 2-way ANOVA. Experiments were cleared through local ethical committees and performed under home office license PPL30/2964. ANOVA, analysis of variance; CFSE, carboxyfluorescein succinimidyl ester; RU, resonance units; SDS-PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis. ***P = .0003; ****P < .0001 (2-way ANOVA, multiple comparisons using Tukey's test).
9E9’s binding specificity was originally determined using CHO cells expressing individual FcγRs, and showed sole specificity for FcγRIV.10 To confirm specificity, we performed surface plasmon resonance (SPR) measurements (Figure 1C), assessing the affinity of 9E9 for murine FcγRI-IV (mFcγRI-IV). In addition to strong reactivity to FcγRIV (Figure 1Civ), 9E9 displayed low-level binding to FcγRII and FcγRIII (Figure 1Cii-iii). To determine whether this reactivity was conferred by 9E9 Fab or Fc, we produced deglycosylated 9E9 (de-gly9E9) using PNGase F, removing the sugar residues at ASN-297 critical for Fc:FcγR interactions.11 After deglycosylation, FcγRII and FcγRIII binding (Figure 1Cii-iii) was lost whereas binding to FcγRIV remained (Figure 1Civ), demonstrating that binding of native 9E9 to FcγRII and FcγRIII was via the Fc.
We considered the possibility that 9E9, bound to FcγRIV could bind (and block) other coexpressed FcγRs. To test this, we used wild-type (WT), FcγRI−/−, or FcγRIII−/− mice in combination with de-gly9E9 and assessed B-cell depletion (Figure 1D). Figure 1B demonstrated that blocking FcγRIV with 9E9 in FcγRI−/− mice abrogated depletion. In contrast, blocking FcγRIV with de-gly9E9 (Figure 1D) had no effect on depletion in WT, FcγRI−/−, or FcγRIII−/− mice. These data indicate that previous observations regarding the key role of FcγRIV may have been overstated as a result of unanticipated multiple FcγR blockade.
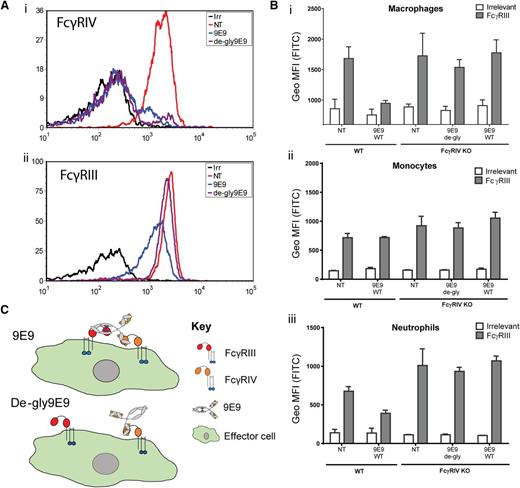
To examine FcγR availability following 9E9 or de-gly9E9 blockade, we examined FcγR expression on myeloid cells following Ritm2a treatment in vivo. On macrophages, neutrophils, and monocytes treated with 9E9 or de-gly9E9, FcγRIV was blocked as expected (Figure 2Ai; supplemental Figure 1, available on the Blood Web site). However, FcγRIII was also blocked on macrophages and neutrophils by 9E9 (Figure 2Aii; supplemental Figure 1). Importantly, de-gly9E9 treatment did not reduce detection of FcγRIII. These data suggested that 9E9 bound to and blocked FcγRIV but also blocked FcγRIII through its Fc. To confirm these effects were not due to high levels of blocking mAb, we titrated 9E9 and found that FcγRIII blockade accompanied that of FcγRIV on macrophages and neutrophils at all concentrations above 12.5 µg (supplemental Figure 2).
Engagement of FcγRIV is critical to the blocking of FcγRIII by 9E9. (A) Representative histograms showing detectable FcγRIV (i) or FcγRIII (ii) on neutrophils isolated from mice in the adoptive transfer studies using either 9E9 or de-gly9E9. Splenocytes were incubated with anti-mLy-6G (PEcy7), Ly6C (PerCP), CD11b (PE), F4/80 (APC), and anti-mFcγR (FITC). Antibodies to detect FcγRIII and FcγRIV were AT152-4 F(ab′)2 and 9E9, respectively. Samples were opsonized for 30 minutes on ice before washing, RBC lysis, and analysis on a Becton Dickinson FACSDiva II flow cytometer. (B) WT or FcγRIV−/− C57BL/6 mice were injected IP with either 9E9 or de-gly9E9 (50 µg). Three to 4 hours later, splenocytes were harvested and the level of detectable FcγRs measured on myeloid subsets, (i) macrophages, (ii) monocytes, and (iii) neutrophils, as in panel A. (C) Schematic diagram of the proposed mechanism by which 9E9 blocks FcγRIII (N = 3). APC, allophycocyanin; FITC, fluorescein isothiocyanate; IP, intraperitoneally; irr, irrelevant control; KO, knockout; MFI, mean fluorescence intensity; NT, nontreated; PE, phycoerythrin; PerCP, peridinin chlorophyll; RBC, red blood cell.
Engagement of FcγRIV is critical to the blocking of FcγRIII by 9E9. (A) Representative histograms showing detectable FcγRIV (i) or FcγRIII (ii) on neutrophils isolated from mice in the adoptive transfer studies using either 9E9 or de-gly9E9. Splenocytes were incubated with anti-mLy-6G (PEcy7), Ly6C (PerCP), CD11b (PE), F4/80 (APC), and anti-mFcγR (FITC). Antibodies to detect FcγRIII and FcγRIV were AT152-4 F(ab′)2 and 9E9, respectively. Samples were opsonized for 30 minutes on ice before washing, RBC lysis, and analysis on a Becton Dickinson FACSDiva II flow cytometer. (B) WT or FcγRIV−/− C57BL/6 mice were injected IP with either 9E9 or de-gly9E9 (50 µg). Three to 4 hours later, splenocytes were harvested and the level of detectable FcγRs measured on myeloid subsets, (i) macrophages, (ii) monocytes, and (iii) neutrophils, as in panel A. (C) Schematic diagram of the proposed mechanism by which 9E9 blocks FcγRIII (N = 3). APC, allophycocyanin; FITC, fluorescein isothiocyanate; IP, intraperitoneally; irr, irrelevant control; KO, knockout; MFI, mean fluorescence intensity; NT, nontreated; PE, phycoerythrin; PerCP, peridinin chlorophyll; RBC, red blood cell.
Because 9E9 binds FcγRII and FcγRIII with low affinity (Figure 1C), we hypothesized that clustering of 9E9 on the cell surface was required for subsequent Fc blocking of FcγRIII. As monocytes do not express appreciable levels of FcγRIV, this would explain why FcγRIII detection was not reduced on these cells after 9E9 blockade. To test this, we administered 9E9 or de-gly9E9 to FcγRIV−/− mice and assessed FcγRIII binding. In the absence of FcγRIV, 9E9 and de-gly9E9 did not reduce detection of FcγRIII on macrophages (Figure 2B).
Taken together, these results demonstrate that 9E9 blocking studies overemphasize the contribution of FcγRIV when the effector cells express multiple FcγRs as illustrated in Figure 2C. Regarding which FcγRs are critical for depletion of B cells with anti-CD20 mAbs, our data support the view that all activatory FcγRs (including FcγRIII) are capable of mediating depletion. This conclusion is in agreement with data showing that at high tumor doses FcγRIV alone is not sufficient to confer significant survival and that either FcγRI or FcγRIII in conjunction with FcγRIV is needed.12 Clearly, these results have implications for the interpretation of previous data and the design of future studies.
The online version of this article contains a data supplement.
Authorship
Acknowledgments: The authors thank Prof Jeffrey V. Ravetch, Prof Mark J. Shlomchik, and Dr J. Sjef Verbeek for provision of 9E9 antibody, hCD20 Tg mice, and FcγRI−/− and FcγRIII−/− mice, respectively.
This work was supported by Bloodwise grants 10055 and 12050.
Contribution: T.R.W.T. helped design the research, performed experiments, analyzed results, produced figures, and wrote the manuscript; C.I.M. and R.R.F. performed experiments and analyzed results; A.L.T. generated critical reagents; and M.S.C. and S.A.B. designed and supervised the research, analyzed results, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mark S. Cragg, Antibody and Vaccine Group, Cancer Sciences Unit, University of Southampton Faculty of Medicine, Southampton, United Kingdom; e-mail: msc@soton.ac.uk; and Stephen A. Beers, Antibody and Vaccine Group, Cancer Sciences Unit, University of Southampton Faculty of Medicine, Southampton, United Kingdom; e-mail: sab@soton.ac.uk.
References
Author notes
M.S.C. and S.A.B. jointly supervised this work.