“There is at bottom only one genuinely scientific treatment for all diseases, and that is to stimulate the phagocytes.” We have come a long way since this declamation by Sir Ralph Bloomfield Bonington in George Bernard Shaw’s 1906 play, The Doctor’s Dilemma. Stimulation of phagocytes is even more relevant for understanding the process of inflammation now, over 100 years later, as knowledge of pro- and anti-inflammatory cytokines and macrophage polarization in infectious and inflammatory diseases and cancer has expanded. In this issue of Blood, Bagaitkar et al add further to the understanding of how inflammation is controlled.1
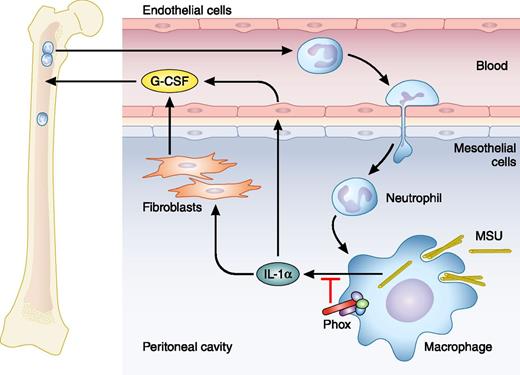
MSU crystals injected i.p. are taken up by macrophages and induce secretion of IL-1α. IL-1α stimulates production of G-CSF (here by fibroblasts and endothelial cells), which causes release of neutrophils from bone marrow to blood and further to tissue. NADPH oxidase (Phox), a normal constituent of macrophages (and neutrophils), reduces the amount of IL-1α secreted from macrophages; consequently, it reduces the production of G-CSF and the mobilization of neutrophils from bone marrow and their recruitment to the site of inflammation, where they may activate macrophages further. Professional illustration by Patrick Lane, ScEYEnce Studios.
MSU crystals injected i.p. are taken up by macrophages and induce secretion of IL-1α. IL-1α stimulates production of G-CSF (here by fibroblasts and endothelial cells), which causes release of neutrophils from bone marrow to blood and further to tissue. NADPH oxidase (Phox), a normal constituent of macrophages (and neutrophils), reduces the amount of IL-1α secreted from macrophages; consequently, it reduces the production of G-CSF and the mobilization of neutrophils from bone marrow and their recruitment to the site of inflammation, where they may activate macrophages further. Professional illustration by Patrick Lane, ScEYEnce Studios.
Inflammation is the body’s response to infection and may cause extensive destruction of tissue whether evoked by infection (such as by Mycobacterium tuberculosis) or whether no infectious organism has (yet) been identified as inducing inflammation (such as in rheumatoid arthritis and inflammatory bowel diseases).
Chronic granulomatous disease (CGD) is a severe inherited immunodeficiency that in its classic forms is caused by mutations in 1 of 4 components that together with a fifth component, p40phox, constitute the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase of activated phagocytes.2 NADPH oxidase consumes oxygen for the delivery of 1 electron to reduce molecular oxygen to the superoxide anion; from this, other reactive oxygen derivatives are formed depending on cell type. In CGD, NADPH oxidase cannot be assembled. The clinical picture is characterized by a profound, but quite narrow, deficiency of microbial killing and by an exaggerated inflammatory response in the form of granuloma and inflammatory bowel disease. Recently, an important aspect of how inflammation is controlled by NADPH oxidase activity was discovered by Campbell et al, who demonstrated that the mere consumption of oxygen by NADPH oxidase causes activation of hypoxia-inducible factor 1 in epithelial cells during inflammation of the gut. This drives a protective response that dampens inflammation and tissue destruction.3
In this issue, Bagaitkar et al demonstrate that the NADPH oxidase inhibits macrophage activation and recruitment of neutrophils in a mouse model of sterile inflammation elicited by intraperitoneal (i.p.) injection of monosodium urate (MSU) crystals, well known to cause painful inflammation in gout.1 Activation of macrophages is driven by recognition of pathogen-associated molecular patterns (PAMPs) or damage (or danger)-associated molecular patterns (DAMPs). PAMPs are structures shared by classes of pathogens, an archetype of which is lipopolysaccharide from gram-negative bacteria, and are recognized largely by Toll-like receptors. DAMPs are structures liberated by tissue damage, such as adenosine triphosphate, DNA, and, as here, uric acid, and sensed by intracellular NLRP3. This leads to formation of a structure called the inflammasome. As the name implies, this is central to inflammation. An intracellular protease, caspase-1, is recruited to the inflammasome, is activated, and converts proinflammatory cytokines, primarily interleukins 1β and 18 (IL-1β and IL-18), from inactive to active proteins that are then secreted and are central activators of inflammation.4
IL-1β has a cousin, IL-1α. They both bind the same receptor on the surface of cells, but in contrast to IL-1β, IL-1α also works intracellularly in the nucleus to control gene expression and does not need processing by caspase-1 to be active.5 It is well established that IL-1α mediates recruitment of neutrophils to sites where DAMPs are generated (eg, from necrotic cells injected i.p.).6
Bagaitkar et al make a very fruitful connection between the exaggerated inflammation in CGD and DAMP-induced inflammation and ask whether NADPH oxidase controls DAMP-induced inflammation. Indeed it does. A CGD mouse has an exaggerated inflammatory response to uric acid crystals or necrotic cells injected into the peritoneum, recruiting double the number of neutrophils into blood and from there into the peritoneum, compared with wild-type (WT) mice. The increased influx of neutrophils is solely a consequence of enhanced mobilization of neutrophils from the bone marrow by elevated levels of granulocyte colony-stimulating factor (G-CSF); this is caused by elevated levels of IL-1α and could be eliminated by antibody preventing IL-1α from binding to its receptor (see figure). In contrast to IL-1β, IL-1α is expressed by many cells in addition to macrophages, but the elevated levels of IL-1α in the CGD mouse originate from macrophages, as nicely demonstrated by studies of chimeric mice, that is, WT mice transplanted with bone marrow from CGD mice and allowed to repopulate tissue with macrophages from the CGD bone marrow. An important additional observation made was that depletion of CGD neutrophils (antibody mediated) reduced the number of activated macrophages in the peritoneum after DAMP challenge. This demonstrates that a cycle exists between macrophages and neutrophils to propagate inflammation if not dampened by NADPH oxidase.
This study makes us reexamine the control of inflammation, especially in CGD patients. It raises the question of why the inability to assemble NADPH oxidase leads to more secretion of IL-1α.
Conflict-of-interest disclosure: The author declares no competing financial interests.