In this issue of Blood, Chopra et al provide convincing evidence that tumor necrosis factor (TNF)-like weak inducer of apoptosis (TWEAK) ligand acting through its receptor, fibroblast growth factor-inducible 14 (Fn14), is crucial to the intestinal apoptosis seen in graft-versus-host disease (GVHD) and associated mortality.1
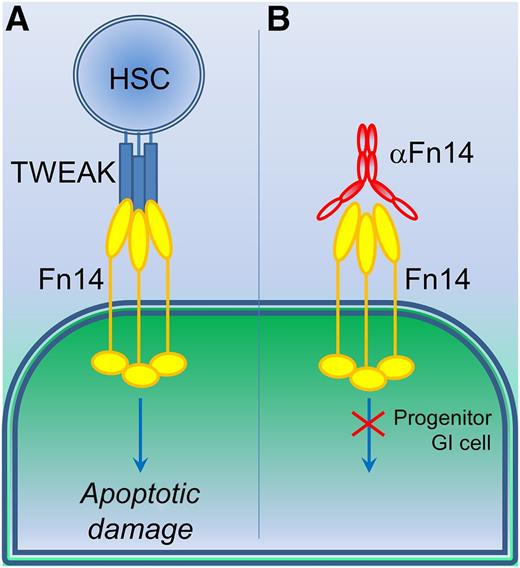
The use of TWEAK and Fn14 reagents to counter GVHD damage. (A) Currently, allogeneic transplant of hematopoietic stem cells (HSC) following whole body irradiation during treatment for leukemia or lymphoma blood cancer would elicit apoptotic GI cell damage through TWEAK stimulation of Fn14 receptors. (B) Use of an anti-Fn14 IgG blocking antibody (αFn14) prevents TWEAK-induced cellular damage, particularly in progenitor GI cells, which overexpress Fn14 receptor.
The use of TWEAK and Fn14 reagents to counter GVHD damage. (A) Currently, allogeneic transplant of hematopoietic stem cells (HSC) following whole body irradiation during treatment for leukemia or lymphoma blood cancer would elicit apoptotic GI cell damage through TWEAK stimulation of Fn14 receptors. (B) Use of an anti-Fn14 IgG blocking antibody (αFn14) prevents TWEAK-induced cellular damage, particularly in progenitor GI cells, which overexpress Fn14 receptor.
Clinically, GVHD is difficult to manage, particularly in leukemia or lymphoma patients who have undergone hematopoietic stem cell transplantation (HSCT). This is in part due to limitations of the understanding of GVHD disease mechanisms, or associated inflammatory cell damage of a patient’s systemic tissues from the transplanted cells, including immune-reactive apoptotic damage of the gastrointestinal (GI) wall. Attempts to control this damage center around thwarting systemic proinflammatory cytokine actions, which results in a greater propensity for patient side effects. Treatment is with a range of anti-inflammatory drugs to suppress the patient’s immune system, just when it is needed the most. Chronic proinflammatory damage can often be as dangerous to the patient as the original blood cancer he or she is being treated for, with significant morbidity and mortality. Clearly, there is an unmet clinical need for more targeted therapies to counter side effects from HSCT therapy. Understanding proinflammatory processes that damage the GI cell wall from such therapies would lead to a better understanding of apoptotic damage processes underway in HSCT patients but may also provide critical clues in the fight against other inflammatory bowel disorders and conditions.
Understanding a disease and its damaging mechanisms is one thing. Being able to pharmacologically counter that disease or any therapy- inducing side effects is quite another. Nonspecific anti-inflammatory agents and steroids are the only real alternatives at present. In recent decades, advances in disease understanding have far outstripped the pharmaceutical and biotechnological industries’ capability to oppose these diseases in the clinic. One bright light for the triumph of pharmacology over disease is therapies developed to affect chronic inflammatory conditions that involve the pleiotropic cytokine TNF. Since the introduction of infliximab and adalimumab humanized monoclonal antibodies and etanercept TNF-receptor fusion protein, the treatments of chronic inflammatory conditions, including rheumatoid arthritis, Crohn disease, and ulcerative colitis, have been markedly improved by the clinical instigation of these TNF-blocking agents. The breathtaking success of these agents has caused the industry to focus its attention onto other members of the TNF-receptor superfamily. Results with a variety of TNF-receptor superfamily blocking agents have shown mixed success thus far. Taking a more long-term view of the clinical successes of these agents suggests certain TNF-receptor family modulating agents will indeed be introduced into the clinic, but the disease-specificity of the TNF receptor and the context-specificity of these therapies still need to be worked out. A more tissue-specific method may be necessary. Also, a more personalized medicine approach may be fundamental to improving the patient’s clinical score and associated toxic side effects.
TWEAK is a good example of the TNF-receptor superfamily that may be useful in certain clinical contexts, but its potential remains relatively unknown. TWEAK is itself a multifunctional cytokine with many potential cellular activities including proliferation, inflammation, migration, angiogenesis, and apoptosis. TWEAK (also known as TNFSF12/APO3L/CD255) was discovered and termed a TNF-like pro-apoptotic agent in human HT-29 colon carcinoma cells.2 The receptor for TWEAK was assumed to be a member of the TNF-receptor superfamily, and after some initial assumptions around death receptor-3, the receptor for TWEAK was found to be Fn14, an unusual small-cell surface receptor with lower sequence similarity to TNF receptors, but nonetheless a receptor containing a signature TNF-receptor–associating factor domain.3,4 Fn14 was found to be highly expressed in many cells of nonlymphoid lineage and was particularly well expressed in epithelial and mesenchymal progenitor cells. Fn14 expression is upregulated in tissue damage settings in response to a variety of insults including hypoxia, oxidative stress, and chemical/mechanical injury. Given Fn14’s original identification from a fibroblast growth factor-induced screen,5 its enhanced expression in cancer tissue (including hepatoma, glioma, and non–small-cell lung cancer) is not entirely surprising.6-8 The TWEAK–Fn14 system has a pivotal role in inflammatory bowel disease (IBD) models9,10 and intestinal epithelial cell death induced by irradiation and IL-13, both key early events in IBD pathogenesis, as well as mucosal epithelial barrier breakdown necessary for pathogen-induced immune cell molecular patterning. Fn14’s more limited tissue distribution than TNF receptors and its enhanced expression in progenitor cells make it an intriguing receptor that could prove very useful in combatting specific tissue damage and certain cancer types.
Similar to the pharmacologic targeting of TNF and its receptors, a variety of recombinant soluble TWEAK variants and TWEAK- or Fn14-specific antibodies have been generated, with some currently under evaluation in clinical trials. Time will tell about the eventual success of these TWEAK–Fn14 modulating agents, but their future looks hopeful. Here, Chopra et al1 eloquently use such TWEAK–Fn14 regulating agents in a mouse model of GVHD to much success. Using lethally irradiated mice receiving allogeneic HSCT as a model of GVHD and graft-versus-leukemia/lymphoma (GVL) effect, they found that an Fn14-blocking antibody-dependent cellular cytotoxicity-deficient human immunoglobulin G1 significantly reduced severity of GVHD GI damage without any negative effects on the required GVL activity (see figure). Importantly, Fn14-blocking antibodies can prevent allogeneic HSCT-induced GVHD damage without inhibiting the desired GVL responses. In HSCT, cotreatments need to inhibit proinflammatory effects from the transplanted cells while maintaining desirable disease-reversing responses from those same cells. Currently, general anti-inflammatory co-treatments serve to inhibit both GVHD and GVL. Fn14-blocking antibodies (1) improved the GI apoptotic scoring of the mice as a mark of acute GVHD severity, (2) maintained the anti-lymphoma responses, and (3) significantly improved the disease score and overall survival rates of the model animals.
TNF receptors play many key roles in healthy tissues and can be key in pathological responses observed in a range of chronic immune diseases. Not only does TWEAK/Fn14-specific blockade highlight the specific nature of some members of the TNF-receptor superfamily in certain human conditions, but it heralds that this ligand and receptor could be rather useful in controlling GVHD damage without impinging on the positive effects of the HSCT treatment itself. Furthermore, agents that control the TWEAK/Fn14 system may also prove to be clinically useful in other chronic inflammatory disorders, particularly those involving the GI tract.
Conflict-of-interest disclosure: The author declares no competing financial interests.