Abstract
Waldenström macroglobulinemia (WM) is a B-cell neoplasm manifested by the accumulation of clonal immunoglobulin (Ig)M-secreting lymphoplasmacytic cells. MYD88 and CXCR4 warts, hypogammaglobulinemia, infections, myelokathexis syndrome-like somatic mutations are present in >90% and 30% to 35% of WM patients, respectively, and impact disease presentation, treatment outcome, and overall survival. Familial predisposition is common in WM. Asymptomatic patients should be observed. Patients with disease-related hemoglobin <10 g/L, platelets <100 × 109/L, bulky adenopathy and/or organomegaly, symptomatic hyperviscosity, peripheral neuropathy, amyloidosis, cryoglobulinemia, cold-agglutinin disease, or transformed disease should be considered for therapy. Plasmapheresis should be used for patients with symptomatic hyperviscosity and before rituximab for those with high serum IgM levels to preempt a symptomatic IgM flare. Treatment choice should take into account specific goals of therapy, necessity for rapid disease control, risk of treatment-related neuropathy, immunosuppression and secondary malignancies, and planning for future autologous stem cell transplantation. Frontline treatments include rituximab alone or rituximab combined with alkylators (bendamustine and cyclophosphamide), proteasome inhibitors (bortezomib and carfilzomib), nucleoside analogs (fludarabine and cladribine), and ibrutinib. In the salvage setting, an alternative frontline regimen, ibrutinib, everolimus, or stem cell transplantation can be considered. Investigational therapies under development for WM include agents that target MYD88, CXCR4, BCL2, and CD27/CD70 signaling, novel proteasome inhibitors, and chimeric antigen receptor-modified T-cell therapy.
Introduction
The cases presented in Table 1 revealed Waldenström macroglobulinemia (WM), a B-cell neoplasm resulting from the accumulation of clonal lymphoplasmacytic cells secreting a monoclonal immunoglobulin (Ig)M protein.1,2 WM patients can present with a wide array of symptoms and findings that impact diagnostic workup and treatment. MYD88 and CXCR4 WHIM-like somatic mutations are present in >90% and 30% to 35% of WM patients, respectively, but are absent or rare in other IgM secreting B-cell malignancies.3-12 More than one half of individuals with IgM-secreting monoclonal gammopathy of unknown significance harbor the MYD88L265P mutation, suggesting its role as an early oncogenic driver.4-6 Somatic CXCR4 mutations are similar to those found in the germ line of WHIM (warts, hypogammaglobulinemia, infections, myelokathexis) syndrome and nearly always are present in WM patients with MYD88L265P.9,13 Both nonsense (CXCR4WHIM/NS) and frameshift CXCR4 mutations (CXCR4WHIM/FS) occur in WM patients. MYD88 and CXCR4 mutations may impact disease presentation, treatment outcome, and/or survival.3-14 Low tumor burden and serum IgM levels are associated with wild-type MYD88 (MYD88WT) disease, whereas MYD88L265P patients with CXCR4WHIM mutations have higher bone marrow (BM) disease burden.13 High serum IgM levels including presentation with hyperviscosity crisis have been observed in patients with CXCR4WHIM/FS tumor mutation status.11,13 Ibrutinib response (discussed below) is adversely impacted by MYD88WT and CXCR4WHIM status. In 1 study, overall survival was lower in patients with MYD88WT disease but was unaffected by CXCR4 mutation status.13
Clinical features
The clinical and laboratory findings for a large series of newly diagnosed patients who presented to the WM clinic at the Dana Farber Cancer Institute (a tertiary referral center) are depicted in Table 2. Splenomegaly and lymphadenopathy (as with case 2) are uncommon at initial presentation (15-20%), although at later stages, extramedullary disease is more common (up to 60% of patients).15-17 WM-related morbidity may be manifested by tumor cell infiltration and/or by the physicochemical and immunologic properties of the monoclonal IgM protein produced by WM cells (Table 3).18-20
Diagnostic workup
History taking
Familial predisposition is strong in WM.21-23 Familial WM patients are younger, have a higher disease burden, and show lower response rates and progression-free survival (PFS) with non–proteasome inhibitor-based therapy.24 Hepatitis C exposure as a WM predisposition remains controversial, although it is associated with type II cryoglobulinemia.25-27 A thorough review of systems should be performed given a wide range of WM-related morbidity that exists, which can impact the diagnostic workup and treatment undertaken (Table 4).
Laboratory studies
To establish the WM diagnosis, an IgM monoclonal protein and BM lymphoplasmacytic lymphoma (LPL) infiltration must be present.1 No minimum serum IgM or BM infiltration level is required to diagnose WM, because patients can be symptomatic and need treatment at low serum IgM (<1000 mg/dL) levels or BM disease involvement.28 Laboratory and clinical evaluations that can be performed in the initial workup of all WM patients and those with particular disease-related features are presented in Table 4.
Treatment approaches to WM
Management of the asymptomatic WM patient
Patients with disease-related hemoglobin level (<10 g/dL), platelet count <100 × 109/L, or symptomatic disease manifested by hyperviscosity, amyloidosis (particularly if there is heart involvement), cryoglobulinemia, cold agglutinemia, extramedullary disease including central nervous system (Bing-Neel syndrome) involvement, moderate severe or advancing paraprotein-related peripheral neuropathy (PN), or disease transformation should be considered for therapy.29,30 Treatment initiation should not be based on serum IgM levels per se, although at higher serum IgM levels (>6000 mg/dL), empiric treatment may be appropriate to prevent hyperviscosity-related injury.
Management of the symptomatic WM patient
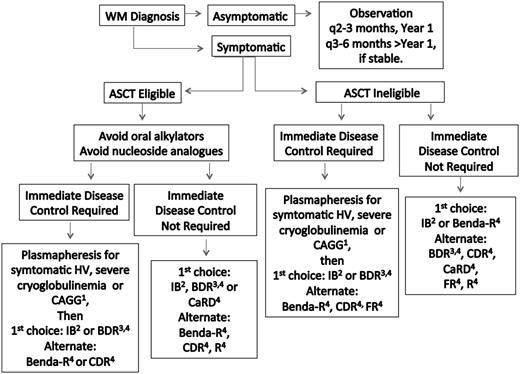
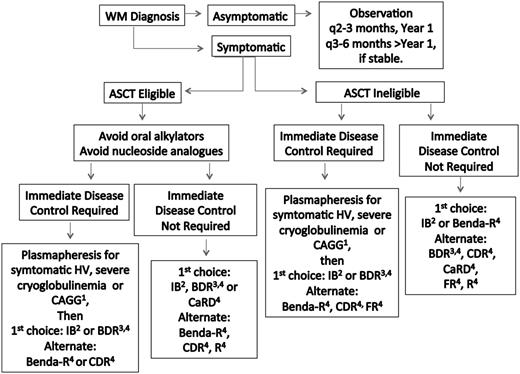
Treatment choice should take into account specific goals of therapy, necessity for rapid disease control, risk of treatment-related neuropathy, immunosuppression, secondary malignancies, and potential for future autologous stem cell transplantation (ASCT). Hepcidin-related iron deficiency is common in WM patients.31 In select patients with low disease burden, symptomatic anemia, and depressed iron saturation levels (ie, <10-12%) unrelated to gastrointestinal bleeding, parenteral iron administration may be beneficial.32 If unresponsive to parenteral iron, chemotherapy can then be initiated. Plasmapheresis should be considered for patients in need of immediate paraprotein control (discussed below) and before rituximab if serum IgM is ≥4000 mg/dL to preempt a symptomatic IgM flare.30,33 A suggested algorithm for the primary therapy of WM is provided in Figure 1. Continuous oral alkylator therapy (such as chlorambucil) and nucleoside analogs should be avoided in ASCT candidates to prevent stem cell damage, as well as in younger patients (<65-70 years), given their association with secondary malignancies and/or disease transformation.30,33-37
Guide to the primary therapy of WM. HV, hyperviscosity; CRYO, cryoglobulinemia; CAGG, cold agglutinemia; BDR, bortezomib, dexamethasone, rituximab; Benda-R, bendamustine, rituximab; CaRD, carfilzomib, rituximab, dexamethasone; CDR, cyclophosphamide, dexamethasone, rituximab; FR, fludarabine, rituximab; IB, ibrutinib; R, rituximab. 1Consider blood warmers with plasmapheresis in patients with cryoglobulinemia or cold agglutinemia to avoid cryoprecipitation or agglutination. 2Can be considered in patients who are not wild type for MYD8869 and for those patients without bulky adenopathy or Bing-Neel syndrome. 3For patients requiring immediate disease control, consider twice weekly dosed bortezomib for 1 to 2 cycles, and then if the patient is stable, switch to weekly bortezomib to reduce risk of treatment related peripheral neuropathy. In patients not requiring immediate disease control, the use of weekly dosed bortezomib is preferable. Bortezomib should be avoided in patients with disease-related neuropathy. Bortezomib should be held for grade ≥2 treatment-related neuropathy. Acyclovir and famotidine (or equivalent) are strongly recommended for patients on proteasome inhibitor therapy. 4Rituximab should be held in patients with symptomatic HV, severe CRYO or CAGG, and in asymptomatic patients with serum IgM >4000 mg/dL to avoid an IgM flare and potentiation of symptoms. Ofatumumab may be considered for rituximab-intolerant patients. Consider maintenance rituximab for patients responding to a rituximab-containing regimen. See text for suggested dosing, cycles, and scheduling of therapy. A clinical trial should be considered whenever possible.
Guide to the primary therapy of WM. HV, hyperviscosity; CRYO, cryoglobulinemia; CAGG, cold agglutinemia; BDR, bortezomib, dexamethasone, rituximab; Benda-R, bendamustine, rituximab; CaRD, carfilzomib, rituximab, dexamethasone; CDR, cyclophosphamide, dexamethasone, rituximab; FR, fludarabine, rituximab; IB, ibrutinib; R, rituximab. 1Consider blood warmers with plasmapheresis in patients with cryoglobulinemia or cold agglutinemia to avoid cryoprecipitation or agglutination. 2Can be considered in patients who are not wild type for MYD8869 and for those patients without bulky adenopathy or Bing-Neel syndrome. 3For patients requiring immediate disease control, consider twice weekly dosed bortezomib for 1 to 2 cycles, and then if the patient is stable, switch to weekly bortezomib to reduce risk of treatment related peripheral neuropathy. In patients not requiring immediate disease control, the use of weekly dosed bortezomib is preferable. Bortezomib should be avoided in patients with disease-related neuropathy. Bortezomib should be held for grade ≥2 treatment-related neuropathy. Acyclovir and famotidine (or equivalent) are strongly recommended for patients on proteasome inhibitor therapy. 4Rituximab should be held in patients with symptomatic HV, severe CRYO or CAGG, and in asymptomatic patients with serum IgM >4000 mg/dL to avoid an IgM flare and potentiation of symptoms. Ofatumumab may be considered for rituximab-intolerant patients. Consider maintenance rituximab for patients responding to a rituximab-containing regimen. See text for suggested dosing, cycles, and scheduling of therapy. A clinical trial should be considered whenever possible.
Frontline therapy of WM
For symptomatic, untreated WM patients not requiring immediate disease control as exemplified in case 1, options include rituximab alone or rituximab with alkylators (bendamustine and cyclophosphamide), proteasome inhibitors (bortezomib and carfilzomib), nucleoside analogs (fludarabine and cladribine), and ibrutinib. Rituximab alone is well suited for more indolent WM patients, ie, those with mild-moderate symptomatic anemia or PN (as for case 1) and those for whom more aggressive chemotherapy is inappropriate. Rituximab monotherapy should not be used in a patient in whom rapid responses are required, because response times can be slow. In addition, a rituximab-induced IgM flare (defined as ≥25% increase above baseline serum IgM level) occurs in 40-60% of WM patients and can provoke or accentuate IgM-related morbidity including symptomatic hyperviscosity, PN, cold agglutinemia, and cryoglobulinemia.38-42 Rituximab monotherapy produces overall response rates (ORRs) of 25% to 40% with standard (375 mg/m2 per week for 4 weeks) therapy and 40% to 60% with extended (375 mg/m2 per week at weeks 1-4 and 12-16) therapy, with median PFS estimates of 16 to 29+ months.43-45 Rituximab-related infusion reactions are common in WM patients, and administration of oral dexamethasone (10 mg) and famotidine (20 mg) the night before rituximab administration in addition to standard hypersensitivity prophylaxis may help reduce these events. Despite prophylaxis, progressive rituximab intolerance is commonly encountered in WM, and ofatumumab may successfully be administered in place of rituximab.46,47
For untreated WM patients experiencing nonemergent paraprotein-related morbidity, a proteasome inhibitor-containing regimen such as bortezomib, dexamethasone, and rituximab (BDR) or carfilzomib, rituximab, and dexamethasone (CaRD) is ideal.48-50 Proteasome inhibitor-containing therapy can also be considered for myelosuppressed patients, although rarely, bortezomib causes thrombocytopenia. In younger patients for whom avoidance of alkylator-based therapy is desired to reduce the risk of secondary malignancies, proteasome inhibitor-based therapy (or ibrutinib as discussed below) can be considered. Bortezomib is best avoided in patients with paraprotein-related neuropathy as in case 1, given the risk for treatment-related neuropathy. For BDR, weekly administration of bortezomib reduces but does not abrogate treatment-related neuropathy.49 There are no data on subcutaneous bortezomib use in WM patients, but this can be considered given the lower neuropathy risk observed with this method of administration in myeloma patients.51 Regardless of route for administration, a close watch for bortezomib-related neuropathy should be undertaken, and the drug should be held for grade ≥2 neuropathy. For weekly BDR, bortezomib can be dosed at 1.6 mg/m2 per week with dexamethasone (20 mg/week) for 12 to 16 weeks, based on patient tolerance. Rituximab can be introduced if the baseline serum IgM is <4000 mg/dL, or when such a level is achieved on weekly bortezomib and dexamethasone alone. Plasmapheresis to lower the serum IgM to <4000 mg/dL before rituximab can also be considered.30,33 One single dose of rituximab (375 mg/m2 per week) can be given every 3 to 4 weeks as part of the weekly BDR regimen. Serum IgM levels should be carefully monitored to detect a rituximab-related IgM flare. Maintenance can be considered for BDR responders (discussed below).
The CaRD regimen, which is associated with a low incidence of treatment-related neuropathy, can also be considered in nonemergent patients.50 Serum IgA and IgG depletion is pronounced with CaRD, which incorporates an extended maintenance schedule, and can contribute to recurring sinus and bronchial infections. A modified CaRD regimen with 1 rituximab infusion per induction cycle, or an abbreviated maintenance course, may be considered to reduce serum IgA and IgG depletion.50 Rituximab should only be introduced when the serum IgM is <4000 mg/dL. Transient increases in amylase, lipase, and bilirubin commonly occur with CaRD and should be monitored. Carfilzomib should be avoided in patients at risk for cardiomyopathy. With either BDR or CaRD, herpes zoster prophylaxis with oral antiviral therapy (ie, acyclovir 400 mg twice daily) for the duration of active therapy plus 6 months should be given, as well as famotidine (20 mg twice per day) during active therapy to decrease risk of gastrointestinal irritation.
For patients with bulky extramedullary disease, malignant pleural effusions, or heavily impacted BM involvement, alkylator-based (bendamustine or cyclophosphamide) therapy can be considered. Benda-R showed longer (69 vs 29 months) PFS and better tolerance vs cyclophosphamide, adriamycin, vincristine, prednisone, and rituximab (CHOP-R) in WM patients included in a randomized study by the German Study Group Indolent Lymphomas.52 Bendamustine can be administered intravenously at 90 mg/m2 on days 1 and 2 every 4 weeks for 4 to 6 cycles, although 4 cycles is sufficient for most WM patients and may reduce risk of prolonged or late myelosuppression. Bendamustine dose should be reduced in elderly patients (ie, to 60-70 mg/m2 on days 1 and 2 every 4 weeks) and those with diminished renal function. Rituximab should be introduced when the serum IgM level is <4000 mg/dL with Benda-R. A cyclophosphamide-based regimen such as rituximab, oral cyclophosphamide, and dexamethasone (R-CD) can be considered as an alternative to Benda-R.30,33 The ORR with R-CD or similar regimens are 80% to 90%, with a median PFS of 3 years.53-55 R-CHOP or rituximab, cyclophosphamide, vincristine, and prednisone (R-CVP) is not advantageous over R-CD or rituximab, cyclophosphamide, and prednisone (R-CP) and can contribute to more toxicities including treatment-related neuropathy.55 Maintenance therapy following Benda-R or R-CD can be considered in responders (discussed below).
Nucleoside analogs with rituximab and/or cyclophosphamide in untreated WM patients are associated with ORRs of 85% to 95%, with a median PFS of 4 years.56-59 It is unclear whether including cyclophosphamide to a nucleoside analog-containing regimen (such as fludarabine, cyclophosphamide, rituximab [FCR]) extends activity in WM and may contribute to added toxicity. Because of immunosuppression, myelosuppression, impact on stem cell collection, and potential secondary malignancy risks including disease transformation, nucleoside analogs should be cautiously used.34-37,56-59 An exception may be for WM patients with Bing-Neel syndrome, for whom central nervous system responses can be obtained with fludarabine.60 WM patients >70 years of age and non-ASCT candidates may also be candidates for nucleoside analog therapy.30,33 In elderly patients, fewer days of therapy per cycle and fewer treatment cycles may be considered to minimize fludarabine-related toxicity.57 Herpes zoster prophylaxis should be given for the duration of nucleoside analog treatment and for at least 1 year from the end of active nucleoside analog therapy.57 Cytokine support per American Society for Clinical Oncology guidelines should be considered with alkylator or nucleoside analog-based therapies.61
Ibrutinib was recently approved by the US Food and Drug Administration and adopted into National Comprehensive Cancer Network guidelines for symptomatic WM patients.30 Although data on the activity of ibrutinib in WM is based on previously treated patients (discussed below), the number of prior lines of therapy had no impact on its activity.14 Moreover, WM patients with fewer prior therapies showed better PFS and less toxicity. However, patients with MYD88WT had considerably lower response rates and shorter PFS, whereas those with CXCR4 mutations showed slower initial response that improved with prolonged (>6 months) therapy.14 The rapid time to IgM reduction (4 weeks) and absence of a rituximab-like IgM flare effect make the use of ibrutinib particularly attractive for patients with high IgM levels (in whom use of rituximab would be problematic), as well as those with IgM-related morbidity. There are no data on ibrutinib use in patients with Bing-Neel syndrome or bulky adenopathy (>5 cm), although reductions in extramedullary disease occurred in most previously treated patients on ibrutinib. The use of ibrutinib (if available) can be considered in symptomatic frontline patients, although alternative options should be considered for patients with bulky disease and MYD88WT disease. Ibrutinib should be avoided in patients on warfarin, with low platelet counts, and with bleeding.14 Patients with a prior history of arrhythmia may also experience tachyarrythmias while on ibrutinib, and either close monitoring or alternative treatment options should be sought. To minimize bleeding risk associated with ibrutinib, any fish oil supplements should be discontinued, and the drug should be withheld for 3 to 7 days before and for 1 to 3 days after an anticipated invasive procedure.14
Treatment of the WM patient requiring immediate disease control
For WM patients requiring immediate paraprotein control (as in case 2), plasmapheresis should initially be performed.30,33 Typically 2 to 3 sessions of plasmapheresis reduce serum IgM levels by 30% to 60%.62,63 Red blood cell transfusions (if required) should ideally follow plasmapheresis to not aggravate the viscosity load.63 Blood warmers should be used during plasmapheresis in patients with cryoglobulinemia or cold agglutinemia to prevent cryoprecipitation and/or erythrocyte agglutination.
Treatment should be initiated as soon as possible after plasmapheresis, as serum IgM levels will return to baseline in 4 to 5 weeks.63 Ibrutinib (if available) may be ideal in such a setting, given the rapid time to IgM reduction (median 4 and 8 weeks to at least a minor and major response, respectively). If ibrutinib is not available, modified BDR consisting of bortezomib at 1.3 mg/m2 and dexamethasone 20 mg can be given twice weekly (on days 1, 4, 8, and 11 every 21 days) for the first 1 and 2 cycles.49 Once the patient is stabilized, bortezomib (1.6 mg/m2) and dexamethasone (20 mg) can be given weekly, and up to 16 weeks (in total) of therapy can be considered. Rituximab (375 mg/m2 per week) every 3 to 4 weeks can be integrated into BDR once the serum IgM is <4000 mg/dL. In some patients, the serum IgM level may remain unsafely high, and continuance of twice-weekly bortezomib may be required. A close watch for bortezomib-related neuropathy should be maintained, and treatment should be held for grade ≥2 neuropathy. CaRD can also be considered, which is associated with low treatment-related neuropathy risk.50 As an alternative, bendamustine-based therapy (as discussed above) can be considered.52 The serum IgM level should be carefully monitored for a few weeks after rituximab is initiated to monitor for an IgM flare. Maintenance therapy can be considered for responders and is discussed below.
Treatment of paraprotein-related PN
For patients with mild progressive IgM-related PN (such as case 1), rituximab alone can be considered, although its clinical impact remains in question.64-67 Frequently, patients show a hematologic response, without improvement in PN symptoms. The depth of response, and elapsed time between manifestation of PN symptoms and therapy, can be factors.68 In patients with chronic longstanding PN, the goal is to prevent symptomatic progression as myelin regeneration occurs very slowly. For patients with moderate to severe IgM-related PN, combination therapy with rituximab may also be effective.67,68 Ibrutinib has shown symptomatic improvement in WM patients with IgM-related PN that progressed after rituximab and is also reasonable.14 In patients presenting with rapidly evolving IgM-related PN, interim plasmapheresis may be useful before instituting definitive chemotherapy. Steroid or intravenous immunoglobulin (IVIG) use is of little value in the treatment of IgM-related PN.68 Proteasome inhibitor-based therapy can be considered for amyloid or cryoglobulin-related PN, using either weekly dosed bortezomib or carfilzomib to minimize risk of treatment-related neuropathy. As before, proteasome inhibitor therapy should be held for grade ≥2 treatment-related neuropathy. Cryoglobulin-related PN should only be treated if chronic symptoms are present and not for transient, cold-induced episodic events.
Therapy in previously treated WM
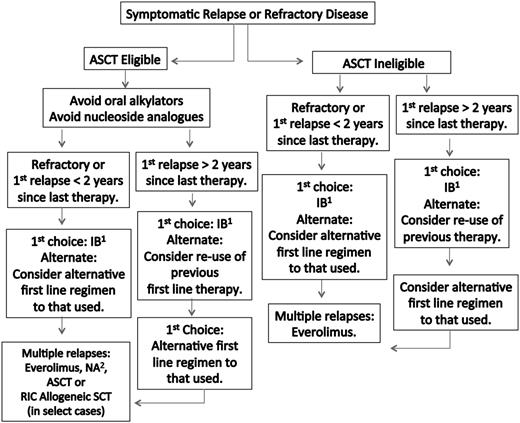
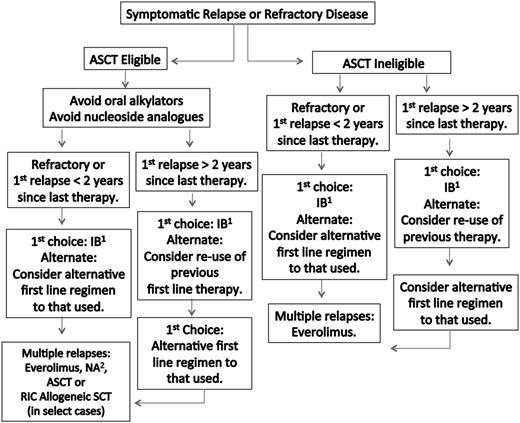
A suggested algorithm for the therapy of previously treated WM patients is provided in Figure 2. The overall response rate for ibrutinib in previously treated WM patients was 91%, with 73% of these patients achieving a major response.14 The 2-year estimate for PFS and overall survival in this study was 69% and 95%, respectively. ORRs and major response rates were highest among patients with the MYD88L265PCXCR4WT genotype (100.0% and 91.2%), followed by MYD88L265PCXCR4WHIM (85.7% and 61.9%) and MYD88WTCXCR4WT (71.4% and 28.6%), respectively. Both major responders among the 7 patients with MYD88WTCXCR4WT were subsequently found to harbor other MYD88 mutations.69
Guide to therapy of previously treated WM. ASCT, autologous stem cell transplant; RIC, reduced intensity allogeneic stem cell transplant; IB, ibrutinib; NA, nucleoside analog-based therapy. 1Can be considered in patients not previously treated with IB, who are not wild type for MYD88, and for those patients without bulky adenopathy or Bing-Neel syndrome. 2In patients being considered for an ASCT, stem cell collection should be undertaken before exposure to a nucleoside analog. Rituximab should be held in patients with symptomatic HV, severe CRYO or CAGG, and in asymptomatic patients with serum IgM >4000 mg/dL to avoid an IgM flare and potentiation of symptoms. Ofatumumab may be considered for rituximab-intolerant patients. Consider maintenance rituximab for patients responding to a rituximab-containing regimen. Bortezomib should be held for grade ≥2 treatment-related neuropathy. See text for suggested dosing, cycles, and scheduling of therapy. A clinical trial should be considered whenever possible.
Guide to therapy of previously treated WM. ASCT, autologous stem cell transplant; RIC, reduced intensity allogeneic stem cell transplant; IB, ibrutinib; NA, nucleoside analog-based therapy. 1Can be considered in patients not previously treated with IB, who are not wild type for MYD88, and for those patients without bulky adenopathy or Bing-Neel syndrome. 2In patients being considered for an ASCT, stem cell collection should be undertaken before exposure to a nucleoside analog. Rituximab should be held in patients with symptomatic HV, severe CRYO or CAGG, and in asymptomatic patients with serum IgM >4000 mg/dL to avoid an IgM flare and potentiation of symptoms. Ofatumumab may be considered for rituximab-intolerant patients. Consider maintenance rituximab for patients responding to a rituximab-containing regimen. Bortezomib should be held for grade ≥2 treatment-related neuropathy. See text for suggested dosing, cycles, and scheduling of therapy. A clinical trial should be considered whenever possible.
Overall, ibrutinib was well tolerated, although neutropenia and thrombocytopenia were more common in heavily pretreated patients. Serum IgA and IgG levels remained unchanged following ibrutinib, and treatment-related infections were infrequent. Patients should be maintained on ibrutinib until evidence of progression or intolerance despite dose reduction. Transient increases in serum IgM frequently occurred when ibrutinib was withheld and did not signify treatment failure as IgM levels declined on reinstitution of therapy.14
Although ibrutinib represents an ideal treatment strategy for relapsed or refractory WM (as in case 3), it is not available in many countries. The use of an alternative first-line agent (as discussed above) can be considered if ibrutinib is not available. In patients for whom ASCT is seriously being considered, exposure to stem cell-damaging agents such as chlorambucil or nucleoside analogs should be avoided, and a non–stem cell toxic approach should be considered if stem cells have not been previously harvested.30,33
Everolimus has been investigated in WM. In previously treated WM patients, the ORRs and major response rates to everolimus were 73% and 50%, respectively, with a median PFS of 21 months.70 Grade ≥3 toxicities, particularly cytopenias, are common with everolimus. Pulmonary toxicity can also occur and can be managed with steroids. Among untreated, symptomatic WM patients, the ORRs and major response rates for everolimus were 72% and 60%, respectively.71 Discordance between serum IgM levels and BM disease response were commonly encountered in the frontline study, which mandated serial BM assessments. Oral ulcerations frequently occurred with everolimus, which could be prevented with an oral dexamethasone swish and spit solution. Because of its toxicity profile and frequent toxicity-related treatment discontinuation, everolimus should be considered for a third or beyond line of therapy in WM patients.
SCT remains an option for salvage therapy in WM, particularly among younger patients who have had multiple relapses or those patients with primary refractory disease. In an European Bone Marrow Transplant Registry study, the 5-year PFS and overall survival were 40% and 69%, respectively, for patients with predominately multi-relapsed or refractory disease who received ASCT.72 The nonrelapse mortality at 1 year was low (4%), and the presence of chemo-refractory disease and number of prior therapies impacted both PFS and overall survival in this study. WM patients with amyloidosis may also benefit from ASCT.73 The outcome of previously treated WM patients who received myeloablative and reduced-intensity allogeneic transplantation was also reported by the European Bone Marrow Transplant Registry.74 Most patients (69%) in this series had chemotherapy-sensitive disease. The ORR was 76%, and the 5-year PFS and overall survival rates were 56% and 62%, respectively. Among patients who received reduced-intensity allogeneic transplantation, similar PFS and overall survival rates were observed (49% and 64%). Nonrelapse mortality at 3 years was high, at 33% and 23% for myeloablative and reduced-intensity allogeneic transplantation, respectively. ASCT, and in very select cases, reduced-intensity allogeneic transplantation, may be considered as appropriate salvage modalities, although the risks and benefits of these modalities should be carefully weighed against other available treatment options including ibrutinib (if available).30,33
Maintenance therapy in WM
Maintenance rituximab therapy may be considered in WM patients responding to a rituximab-containing induction regimen.30,33 In a large retrospective study, postinduction categorical responses improved in 42% of patients receiving maintenance rituximab vs 10% in those on watch-and-wait.75 PFS and overall survival was longer for patients on maintenance rituximab as well. However, more infectious events (usually grade 1 or 2 sinusitis or bronchitis) and lower serum IgA and IgG levels were observed in patients on maintenance therapy. A prospective randomized study examining maintenance rituximab vs observation has been initiated by the German Study Group Indolent Lymphomas, and results are awaited.76
The optimal dosing schedule for maintenance rituximab therapy in WM patients remains unclear. A single rituximab infusion (375 mg/m2) every 3 months for 2 years (as per Van Oers et al77 ) is reasonable over more frequent dosing to minimize rituximab-related serum IgA and IgG depletion.78 Maintenance therapy should be discontinued in WM patients with low serum IgG and/or IgA levels and recurring sino-bronchial infections (≥3-4/year) that require antibiotic support or who experienced a severe infection requiring hospitalization.
Combined maintenance therapy with rituximab and dexamethasone and either bortezomib or carfilzomib has been investigated in WM and can be considered for WM patients who received BDR or CaRD induction, respectively. Serum IgA and IgG depletion may be more pronounced with proteasome-inhibitor containing maintenance regimens, and their levels should be closely monitored.48,50 Shorter courses or longer intervals between cycles may help minimize serum IgA and IgG depletion and can be considered in maintenance strategies that use BDR or CaRD. Neuropathy may also be potentiated with bortezomib-containing maintenance regimens and should be carefully monitored, and treatment should be held for grade ≥2 treatment-related PN.48
Response assessment
Consensus response criteria have been developed as part of the International Workshops on WM.79 A “major response” denotes a ≥50% decrease in serum IgM levels and includes partial and complete responses, whereas the ORR includes minor responders. Durable clinical benefit has been shown in minor responders, whereas deeper categorical responses such as very good partial response or complete response are associated with longer PFS.80,81 Response categories and criteria for progressive disease in WM are summarized in Table 5. Either the serum IgM or the IgM monoclonal spike may be used to track disease burden in WM patients.82 However, gel migration patterns for the IgM paraprotein can vary and confound serial IgM monoclonal spike assessments in many cases.83 In patients with cryoglobulinemia, serum samples should be obtained on warm baths (or equivalent) to minimize cryoprecipitation and serum IgM underestimation. Serum IgM levels can fluctuate with certain biologic agents including rituximab, everolimus, bortezomib, and ibrutinib.14,38,39,71,84 In circumstances when the serum IgM levels appear out of context with clinical impression, a BM biopsy should be considered.
Novel agents for WM
Investigational therapies under development for WM include agents that target MYD88, CXCR4, BCL2, and CD27/CD70 signaling, novel proteasome inhibitors, and chimeric antigen receptor (CAR)-modified T-cell therapy. Idelalisib blocks phosphatidylinositol 3-kinase δ, a growth-promoting transcription factor that is activated by MYD88L265P.85,86 A 70% ORR was observed with idelalisib among 10 previously treated WM patients evaluated in a larger study, and a prospective study with this agent in previously treated WM patients is forthcoming. IMO-8400 is an oligonucleotide that inhibits Toll-like receptors 7, 8, and 9 and induces apoptosis in MYD88L265P WM cells.87 A clinical trial examining IMO-8400 in WM has been initiated. IRAK1/IRAK4 kinases mediate MYD88L265P-directed nuclear factor κB signaling, and their inhibition triggers apoptosis in MYD88L265P-expressing malignant cells.7,88 Moreover, combined BTK and IRAK1/4 inhibition induces synergistic killing of MYD88L265P malignant cells.88 Compounds that inhibit IRAK signaling are under intense preclinical investigation for use in MYD88L265P diseases.89,90 The antiapoptotic factor B-cell lymphoma 2 (BCL-2) is overexpressed in WM cells.91,92 The BCL-2 inhibitor ABT-199 induces apoptosis and shows at least additive antiapoptotic activity against WM cells cotreated with either ibrutinib or idelalisib.93 In a prospective clinical study, 3 of 4 WM previously treated WM patients responded, which included 1 complete response.94
Oprozomib is an oral epoxyketone proteasome inhibitor that is an analog of carfilzomib.95 An ORR of 59% was observed in a phase 2 study in previously treated WM patients.96 Clinical trials examining the oral proteasome inhibitor ixazomib in combination with dexamethasone and rituximab have also been initiated in symptomatic untreated, as well as previously treated WM patients.97,98 Soluble CD27 is released by WM cells and stimulates growth promoting ligands through CD70.99 CD70 is also expressed on WM cells.100 A clinical trial examining a novel human anti-CD70 (ArgenX-70) antibody that mediates ADCC and blocks soluble CD27-CD70 signaling is being initiated in previously treated WM patients.100 In preclinical studies, CXCR4 antagonists blocked SDF (stromal derived factor; CXCL12) rescue of apoptosis mediated by ibrutinib, idelalisib, and other therapeutics.10,101,102 A trial examining ibrutinib with the anti-CXCR4 antibody ulocuplumab (BMS-936564) is being planned in WM patients, given encouraging safety and efficacy data in myeloma patients, as well as in vivo results with ulocuplumab in mice engrafted with CXCR4WHIM-mutated WM cells.10,103 CAR-modified T-cell therapy using a second-generation CAR derived from a CD19-directed antibody fused to the ζ chain of CD3 and the intracellular signaling domain of CD28 (19-28z) has shown robust preclinical activity against WM cells.104 A clinical trial for relapsed or refractory WM patients using 19-28z CAR-modified autologous T cells has been initiated.
Case management
Case 1
Given the nonurgent nature of this WM patient’s presentation, rituximab was started for progressive symptomatic IgM-related neuropathy. The patient did encounter an IgM flare and worsening neuropathic symptoms. He underwent plasmapheresis and was transitioned to CDR, and achieved a partial response with near resolution of his neuropathic symptoms. He completed 2 years of maintenance rituximab therapy and remains in a partial response, without peripheral neuropathy, 5 years later. Under similar circumstances today, ibrutinib (if available) could be considered as an appropriate initial or second-line intervention.
Case 2
This 42-year-old WM patient presented with symptomatic hyperviscosity. The patient also had bulky adenopathy and splenomegaly at the time of presentation. He underwent emergent plasmapheresis, and then received blood transfusions to not aggravate whole blood viscosity levels. Given his young age and need for rapid paraprotein reduction, bortezomib and dexamethasone (without rituximab to avoid an IgM flare) was started. Serum IgM levels were closely monitored after pheresis, and he received additional plasma exchanges when serum IgM exceeded 5000 mg/dL. After 3 cycles of bortezomib and dexamethasone, no response occurred, and he was started on bendamustine. The serum IgM level decreased to 3400 mg/dL after 2 cycles of bendamustine, and no further plasmapheresis was required. Rituximab was then added to bendamustine for 4 additional cycles, with continued close monitoring of serum IgM levels. Maintenance rituximab therapy followed for 2 additional years, and he achieved a very good partial response with complete resolution of all extramedullary disease. Two years after treatment, he remains without progression, although he has required IVIG replacement for recurring sino-bronchial infections associated with treatment-related serum IgA and IgG depletion. Under similar circumstances today, ibrutinib (if available) would represent a very reasonable option to use either as initial or subsequent therapy. The IgG- and IgA-sparing effects associated with ibrutinib may have also prevented the need for IVIG replacement in this patient.
Case 3
This case illustrates a young WM patient refractory to multiple agents. Nucleoside analogs, alemtuzumab, and ASCT were considered, although the patient declined these options because of toxicity concerns. A BM biopsy confirmed persistent WM disease with a MYD88L265PCXCR4WT genotype. He received ibrutinib on a clinical trial and attained a partial response. After therapy, his hematocrit normalized and he remains in a partial response 2.5 years later.
Acknowledgments
This work was supported by Peter S. Bing, M.D., the Linda and Edward Nelson Endowment for Studies into Waldenström Macroglobulienmia, the Kerry Robertson Fund for Waldenstrom’s Research, the Bauman Family Foundation, and the International Waldenstrom’s Macroglobulinemia Foundation.
Authorship
Contribution: S.P.T. wrote the paper.
Conflict-of-interest disclosure: S.P.T. has received active research funding, consulting fees, and/or speaking honoraria from Janssen Pharmaceuticals Inc., Onyx Inc., Pharmacyclics Inc., and Gilead Pharmaceuticals Inc.
Correspondence: Steven P. Treon, Bing Center for Waldenström's Macroglobulinemia, Dana Farber Cancer Institute, M548, 450 Brookline Ave, Boston, MA 02115; e-mail: steven_treon@dfci.harvard.edu.