Key Points
GPVI-dependent platelet binding and activation contribute to seal neutrophil-induced vascular damage in IC-mediated inflammation.
Inflammation represents an uncommon hemostatic situation in which adhesion and activation of single platelets prevent bleeding.
Abstract
Platelets protect vascular integrity during inflammation. Recent evidence suggests that this action is independent of thrombus formation and requires the engagement of glycoprotein VI (GPVI), but it remains unclear how platelets prevent inflammatory bleeding. We investigated whether platelets and GPVI act primarily by preventing detrimental effects of neutrophils using models of immune complex (IC)-mediated inflammation in mice immunodepleted in platelets and/or neutrophils or deficient in GPVI. Depletion of neutrophils prevented bleeding in thrombocytopenic and GPVI−/− mice during IC-mediated dermatitis. GPVI deficiency did not modify neutrophil recruitment, which was reduced by thrombocytopenia. Neutrophil cytotoxic activities were reduced in thrombocytopenic and GPVI−/− mice during IC-mediated inflammation. Intravital microscopy revealed that in this setting, intravascular binding sites for platelets were exposed by neutrophils, and GPVI supported the recruitment of individual platelets to these spots. Furthermore, the platelet secretory response accompanying IC-mediated inflammation was partly mediated by GPVI, and blocking of GPVI signaling impaired the vasculoprotective action of platelets. Together, our results show that GPVI plays a dual role in inflammation by enhancing neutrophil-damaging activities while supporting the activation and hemostatic adhesion of single platelets to neutrophil-induced vascular breaches.
Introduction
Besides being the cellular orchestrators of thrombus formation, platelets are now recognized as major actors of inflammatory reactions. In particular, it is well established that platelets promote leukocyte infiltration in many inflamed tissues and organs by loosening endothelial junctions,1,2 secreting chemokines and serotonin,3,4 and stimulating the expression of adhesion molecules on endothelial plasma membranes.5 Platelets are also known to enhance the formation of prothrombotic antimicrobial and histotoxic neutrophil extracellular traps in response to bacterial and viral infection6,7 and in deep vein thrombosis.8,9 In addition, platelets have been shown to prevent tissue injury during the early phase of inflammatory reactions, thus preventing bleeding in inflamed tissues with high microvessel density such as the skin, kidneys, and lungs.10-14 However, the mechanisms underlying this latter beneficial effect of platelets during inflammation remain unclear. A recent study suggests that the immunoreceptor tyrosine-based activation motif (ITAM)-associated platelet receptors glycoprotein VI (GPVI) and CLEC-2 are involved in the prevention of bleeding by platelets in endotoxin-challenged lungs and in immune complex (IC)-inflamed skin.15 Although this is unlikely to provide the full explanation, there is also strong evidence to suggest that this vasculoprotective action of platelets is independent of their well-known ability to form thrombi at sites of vessel injury.14 Indeed, it has been reported in mouse models of IC-induced vasculitis11 and glomerulonephritis12 that platelet interactions with inflamed microvessels occur before any sign of thrombosis and that prevention of this initial platelet deposition results in accelerated and exacerbated local hemorrhage.12
In addition, there are experimental arguments suggesting that in certain inflammatory situations, platelets can counter the injurious effects of the most abundant type of circulating leukocytes in human, the neutrophil. Renal bleeding and acute renal failure in thrombocytopenic mice subjected to glomerulonephritis was markedly attenuated in mice protected from glomerular neutrophil infiltration by virtue of a genetic deficiency in the leukocyte-specific integrin Mac-1.12 Depletion of neutrophils similarly prevented tumor necrosis factor-α–induced skin bleeding in thrombocytopenic mice.16 Collectively, these observations suggest that the thrombus-independent action of platelets may rely on prevention and/or healing of neutrophil-mediated tissue injury.14 We explored this possibility using mouse models of IC-induced dermatitis and peritonitis and found that platelets enhance rather than diminish neutrophil infiltration and cytotoxic activities during IC-induced inflammation. We further show that although, on one hand, GPVI contributes to these proinflammatory effects of platelets, on the other hand, it also mediates sealing of neutrophil-induced vascular breaches by supporting the early recruitment and adhesion of single platelets to inflamed vessels, as well as platelet secretion and activation, thus contributing to the prevention of inflammatory bleeding.
Materials and methods
Mice
Mice deficient in GPVI−/−17 were backcrossed on a C57Bl/6J background and housed in our animal facility. Their wild-type littermates were used as controls. All animal procedures described in this study were performed using 10-14–week-old mice and were approved by the Animal Care and Use Committee of the Claude Bernard Institute.
Reverse passive Arthus reaction in the skin and peritoneal cavity
Shaved mice were anesthetized with inhalation of isoflurane, and the reverse passive Arthus (rpA) reaction was elicited by subcutaneous injection of rabbit anti-bovine serum albumin (anti-BSA) immunoglobulin G (IgG) (60 µg in 20 µL saline, 2 spots/mouse) immediately followed by IV injection of BSA (50 µg/g mouse) in saline. When the rpA reaction was combined with thrombocytopenia, particular care was taken for the injection of anti-BSA IgG to avoid bleeding from needle insertion.
For induction of the rpA reaction within the peritoneal cavity, 100 µg rabbit anti-BSA IgG in 500 µL saline was injected intraperitoneally immediately followed by IV injection of BSA (50 µg/g mouse) in 50 µL saline. Peritoneal lavage was performed with 2 mL phosphate-buffered saline 1% BSA 4 hours after reaction onset.
Statistical analysis
All data are expressed as mean ± standard error of the mean and were compared by the nonparametric Mann-Whitney U test using GraphPad Prism software (San Diego, CA). P values <.05 were regarded as statistically significant.
Additional methods
Additional methods can be found in supplemental Methods (available on the Blood Web site).
Results
Platelets prevent neutrophil-induced bleeding during IC-mediated inflammation
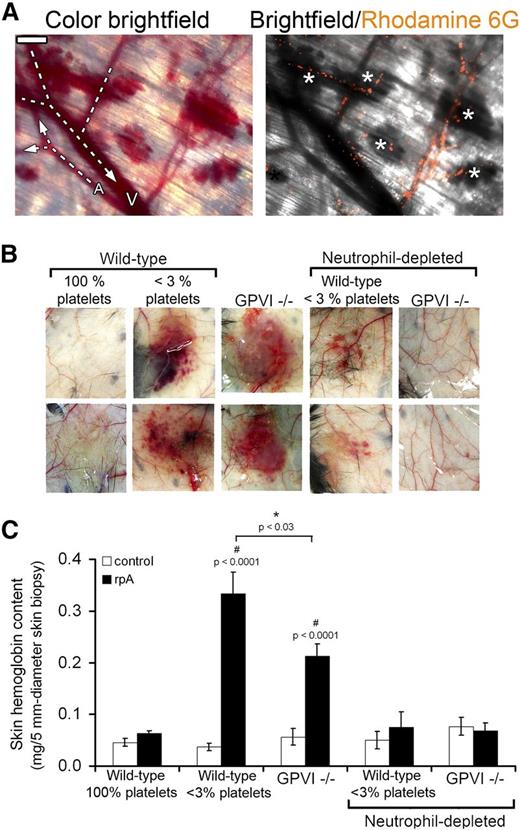
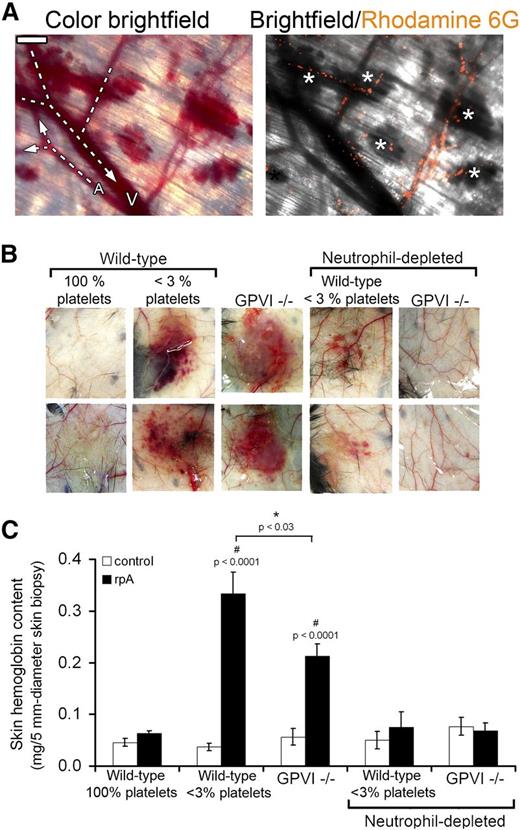
To determine the relationships between leukocyte infiltration and tissue damage during IC-mediated inflammation, we first analyzed the occurrence of skin bleeding relative to leukocyte localization in the skin of thrombocytopenic mice subjected to the cutaneous rpA reaction, a classical experimental model for IC-mediated tissue injury. Intravital observation of skin vessels and rhodamine-6G–labeled leukocytes showed that bleeding during this experimental setting originated abruptly from capillaries and postcapillary venules in which leukocytes accumulated (Figure 1A).
Neutrophils are the cause of skin bleeding in thrombocytopenic and GPVI−/− mice during the cutaneous rpA reaction. (A) Intravital microscopy imaging of the cutaneous rpA reaction in a thrombocytopenic mouse injected with rhodamine-6G for staining of leukocytes. Bright-field color image showing that bleeding originated from capillaries and postcapillary venules (left). Arterioles (A) and venules (V) were identified by whether they collected or distributed blood from/to lower caliber vessels. Merged image of bright-field and corresponding rhodamine-6G image showing that bleeding occurred from vessels in which leukocytes accumulated (white asterisks) (right). Scale bar, 200 µm. (B) Representative images of the inner layer of the skin subjected to the rpA reaction from control (100% platelets), thrombocytopenic (<3% platelets), GPVI−/− mice, and thrombocytopenic and GPVI−/− that were immunodepleted for neutrophils prior to the induction of the rpA reaction. Images were taken 4 hours after inducing the rpA reaction. (C) Hemoglobin content in skin biopsy specimens from control and rpA reaction areas from the various groups of mice, as indicated. n = 6-18 mice per group. # indicates a statistically significant increase (P < .05) in hemoglobin content as compared with control spots from control wild-type mice.
Neutrophils are the cause of skin bleeding in thrombocytopenic and GPVI−/− mice during the cutaneous rpA reaction. (A) Intravital microscopy imaging of the cutaneous rpA reaction in a thrombocytopenic mouse injected with rhodamine-6G for staining of leukocytes. Bright-field color image showing that bleeding originated from capillaries and postcapillary venules (left). Arterioles (A) and venules (V) were identified by whether they collected or distributed blood from/to lower caliber vessels. Merged image of bright-field and corresponding rhodamine-6G image showing that bleeding occurred from vessels in which leukocytes accumulated (white asterisks) (right). Scale bar, 200 µm. (B) Representative images of the inner layer of the skin subjected to the rpA reaction from control (100% platelets), thrombocytopenic (<3% platelets), GPVI−/− mice, and thrombocytopenic and GPVI−/− that were immunodepleted for neutrophils prior to the induction of the rpA reaction. Images were taken 4 hours after inducing the rpA reaction. (C) Hemoglobin content in skin biopsy specimens from control and rpA reaction areas from the various groups of mice, as indicated. n = 6-18 mice per group. # indicates a statistically significant increase (P < .05) in hemoglobin content as compared with control spots from control wild-type mice.
Consequently, we investigated whether depletion of neutrophils, the predominant leukocyte cell type involved in the response to IC challenge,18 could prevent inflammatory skin bleeding in thrombocytopenic wild-type mice and in mice with a genetic deficiency in GPVI (GPVI−/−), an ITAM receptor that was recently reported to contribute to the vasculoprotective action of platelets in the inflamed skin.15 After 4 hours of cutaneous rpA reaction, macroscopically visible petechial skin bleeding had developed in all thrombocytopenic and GPVI−/− mice, whereas no bleeding was observed in their control wild-type littermates (Figure 1B-C). Transfusion of platelets isolated from wild-type mice into GPVI−/− mice (6 × 108 washed platelets per recipient mouse) rescued their bleeding phenotype when subjected to the cutaneous rpA reaction (supplemental Figure 1), confirming that inflammatory bleeding in GPVI−/− mice was due to a platelet defect.
Immunodepletion of circulating neutrophils by >70% using an anti-Ly6G antibody resulted in the almost complete suppression of inflammatory skin bleeding in both thrombocytopenic and GPVI−/− mice (Figure 1B-C). These results show that bleeding in acutely inflamed skin of thrombocytopenic and GPVI−/− mice is caused by neutrophils, thus indicating that platelets limit or repair neutrophil-inflicted injuries during IC-induced inflammation.
Platelets promote the infiltration of neutrophils during IC-mediated inflammation
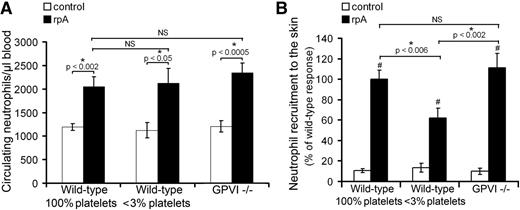
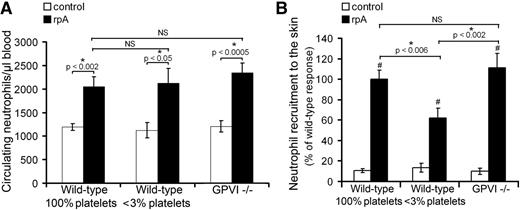
Because for neutrophils to cause damage in a given organ, they first need to reach it, we compared neutrophil mobilization in peripheral blood of control wild-type, thrombocytopenic, and GPVI−/− mice during the cutaneous rpA reaction. In all 3 groups of mice, an increase in the number of circulating neutrophils was observed at 2 hours after initiating the reaction (Figure 2A). There was no difference in the extent of neutrophil mobilization between any of the groups, indicating that systemic mobilization of peripheral neutrophils during IC-mediated inflammation is independent of platelets (Figure 2A).
Platelet contribution to neutrophil peripheral mobilization and recruitment to the skin during the cutaneous rpA reaction. (A) Comparison of the circulating neutrophil count in control wild-type, thrombocytopenic, and GPVI−/− mice before and after induction of the rpA reaction. (B) Quantification and comparison of neutrophil recruitment as assessed by measurement of skin MPO content. # indicates a statistically significant increase (P < .05) in skin MPO content as compared with control spots from control wild-type mice. n = 6-12 mice per group. NS, not significant.
Platelet contribution to neutrophil peripheral mobilization and recruitment to the skin during the cutaneous rpA reaction. (A) Comparison of the circulating neutrophil count in control wild-type, thrombocytopenic, and GPVI−/− mice before and after induction of the rpA reaction. (B) Quantification and comparison of neutrophil recruitment as assessed by measurement of skin MPO content. # indicates a statistically significant increase (P < .05) in skin MPO content as compared with control spots from control wild-type mice. n = 6-12 mice per group. NS, not significant.
We then examined the recruitment of neutrophils to the inflamed skin by measuring the total myeloperoxidase (MPO) content at the reaction site. Induction of a cutaneous rpA reaction led to an increase in total MPO skin content in all groups of mice, indicating the recruitment of neutrophils (Figure 2B). Neutrophil recruitment was comparable in wild-type and GPVI−/− mice but was significantly reduced in thrombocytopenic mice (Figure 2B). Together, these results show that platelets support neutrophil recruitment to the inflamed skin independently of GPVI. They further indicate that tissue damage and bleeding in thrombocytopenic and GPVI−/− mice are not due to exacerbated recruitment of neutrophils at the inflammatory site.
Regulation of neutrophil histotoxic activities by platelets during IC-mediated inflammation
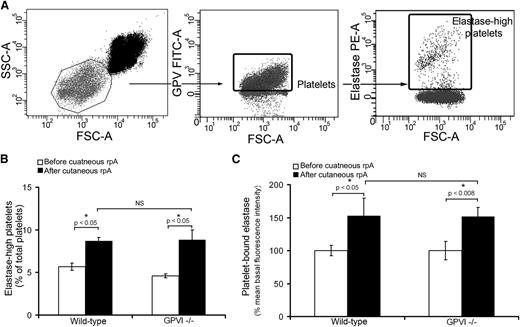
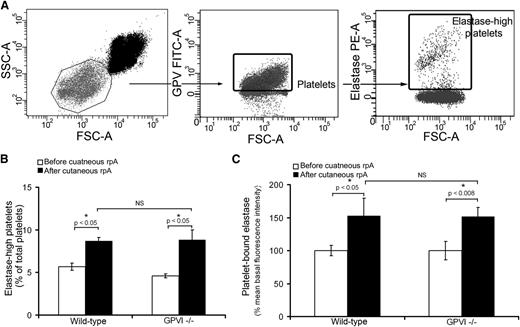
To investigate the possibility of a scavenging role of platelets toward neutrophil-derived proteases, we quantified the level of neutrophil elastase associated with circulating platelets before and 4 hours after inducing the cutaneous rpA reaction. A population of platelets was found to be highly positive for neutrophil elastase, the proportion of which increased significantly from 5.6% at the basal state to 8.7% at 4 hours after induction of the rpA reaction (Figure 3A-B), thus leading to an increase in the mean fluorescence intensity for neutrophil elastase of total platelets (Figure 3C). Similar modifications in elastase levels on circulating platelets were observed in GPVI−/− mice (Figure 3B-C). These results indicate that platelets bind neutrophil elastase during IC-mediated dermatitis, a process that does not require GPVI.
Platelets scavenge neutrophil elastase during IC-mediated inflammation. (A) Flow cytometry dot plots describing the identification of elastase-positive platelets in whole blood. Platelets were identified according to their light-scattering properties (left) and positivity for glycoprotein V (GPV) (middle). A subpopulation of platelets with highly positive elastase was then further identified using a fluorescent polyclonal antibody to neutrophil elastase (right). (B) Comparison of the proportion of elastase-high platelets among total circulating platelets before and 4 hours after inducing the cutaneous rpA reaction in wild-type and GPVI−/− mice. n = 7-8 mice per group. (C) Mean elastase levels on circulating platelets before and 4 hours after inducing the cutaneous rpA reaction in wild-type and GPVI−/− mice. Results are expressed relative to the mean fluorescence intensity for elastase measured on wild-type platelets before the induction of the rpA reaction. n = 7-8 mice per group. FSC-A, forward scatter area; SSC-A, side scatter area.
Platelets scavenge neutrophil elastase during IC-mediated inflammation. (A) Flow cytometry dot plots describing the identification of elastase-positive platelets in whole blood. Platelets were identified according to their light-scattering properties (left) and positivity for glycoprotein V (GPV) (middle). A subpopulation of platelets with highly positive elastase was then further identified using a fluorescent polyclonal antibody to neutrophil elastase (right). (B) Comparison of the proportion of elastase-high platelets among total circulating platelets before and 4 hours after inducing the cutaneous rpA reaction in wild-type and GPVI−/− mice. n = 7-8 mice per group. (C) Mean elastase levels on circulating platelets before and 4 hours after inducing the cutaneous rpA reaction in wild-type and GPVI−/− mice. Results are expressed relative to the mean fluorescence intensity for elastase measured on wild-type platelets before the induction of the rpA reaction. n = 7-8 mice per group. FSC-A, forward scatter area; SSC-A, side scatter area.
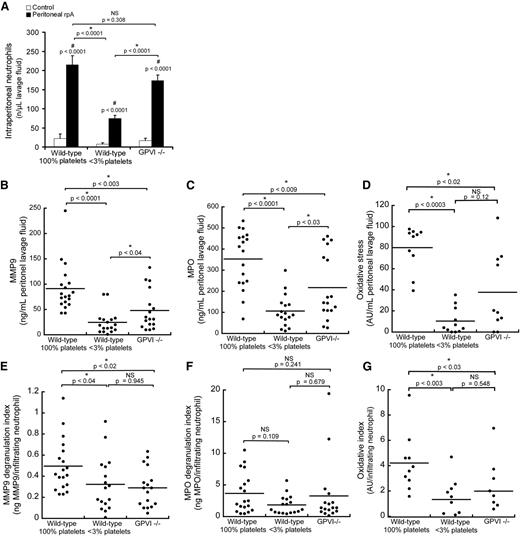
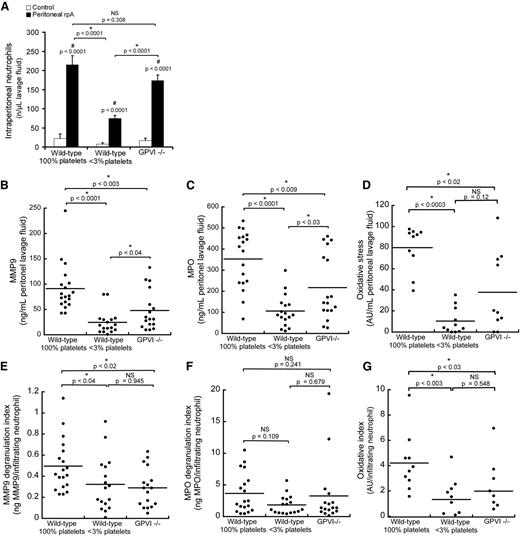
In order to determine the contribution of platelets to the regulation of neutrophil histotoxic compound release during IC-mediated inflammation, we transposed the site of the rpA reaction from the skin to the peritoneal cavity, where neutrophil emigration and released neutrophil granule components can be quantified accurately in lavage fluid. Induction of the rpA reaction within the peritoneal cavity led to a profile of neutrophil recruitment similar to that observed when the reaction was elicited in the skin (Figure 2B), with wild-type and GPVI−/− mice displaying comparable levels of neutrophil recruitment, whereas it was significantly reduced in thrombocytopenic mice (Figure 4A). Based on macroscopic observation of the intraperitoneal organs and collected lavage fluids, IC-mediated inflammation did not cause any hemoperitoneum in either group of mice.
Regulation of neutrophil infiltration, degranulation, and oxidative stress by platelets during the peritoneal rpA reaction. (A) Comparison of neutrophil content in peritoneal lavage fluids from control wild-type, thrombocytopenic, and GPVI−/− mice that were subjected or not to the rpA reaction in the peritoneal cavity. (B-D) Quantification and comparison of MMP-9 (B) and MPO (C) protein levels and of oxidative stress (D) in peritoneal lavage fluids from control wild-type, thrombocytopenic, and GPVI−/− mice subjected to the peritoneal rpA reaction. Each dot corresponds to a mouse. (E-G) MMP-9 (E) and MPO (F) degranulation indexes and oxidative index (G) of mice subjected to the peritoneal rpA reaction Degranulation and oxidative indexes were calculated by dividing levels of MMP-9, MPO, and oxidative activity measured in lavage fluids by the number of infiltrating neutrophils. Each dot represents 1 mouse. NS, not significant.
Regulation of neutrophil infiltration, degranulation, and oxidative stress by platelets during the peritoneal rpA reaction. (A) Comparison of neutrophil content in peritoneal lavage fluids from control wild-type, thrombocytopenic, and GPVI−/− mice that were subjected or not to the rpA reaction in the peritoneal cavity. (B-D) Quantification and comparison of MMP-9 (B) and MPO (C) protein levels and of oxidative stress (D) in peritoneal lavage fluids from control wild-type, thrombocytopenic, and GPVI−/− mice subjected to the peritoneal rpA reaction. Each dot corresponds to a mouse. (E-G) MMP-9 (E) and MPO (F) degranulation indexes and oxidative index (G) of mice subjected to the peritoneal rpA reaction Degranulation and oxidative indexes were calculated by dividing levels of MMP-9, MPO, and oxidative activity measured in lavage fluids by the number of infiltrating neutrophils. Each dot represents 1 mouse. NS, not significant.
Protein levels of matrix metalloproteinase-9 (MMP-9) and MPO were measured in lavage fluids to assess degranulation of early-released gelatinase and late-released azurophilic neutrophil granules, respectively.19 Levels of both MMP-9 and MPO were significantly reduced in lavage fluids from thrombocytopenic and GPVI−/− mice as compared with wild-type mice (Figure 4B-C). Intraperitoneal oxidative stress assessed with the Amplex UltraRed reagent, whose oxidation depends on the availability of both peroxidases and H2O2, was also reduced in thrombocytopenic and GPVI−/− mice (Figure 4D). Nevertheless, because neutrophil infiltration was different in the 3 groups of mice, reduced levels of MMP9, MPO, and oxidative stress could reflect decreased neutrophil numbers rather than impaired neutrophil degranulation or oxidative activity. For this reason, protein levels and global oxidative stress were normalized to the number of infiltrating neutrophils, thus yielding degranulation and oxidation indexes. Similarly to what was observed for the total amount of released MMP9, the degranulation index for MMP-9 was reduced in both thrombocytopenic and GPVI−/− mice, thus indicating that platelets stimulate the release of neutrophil tertiary granules in the rpA reaction (Figure 4E) and that GPVI supports this platelet function. In contrast, the MPO degranulation index was similar between all groups of mice (Figure 4F), thus arguing against a role for platelets in the regulation of neutrophil azurophilic granule release during the rpA reaction. As for MMP-9, the intraperitoneal oxidative index of thrombocytopenic and GPVI−/− mice was decreased, indicating that platelets stimulate neutrophil oxidative activity during the rpA reaction in a GPVI-dependent manner (Figure 4G).
Platelets seal neutrophil-inflicted breaches to vascular integrity during IC-mediated skin inflammation in a GPVI-dependent manner
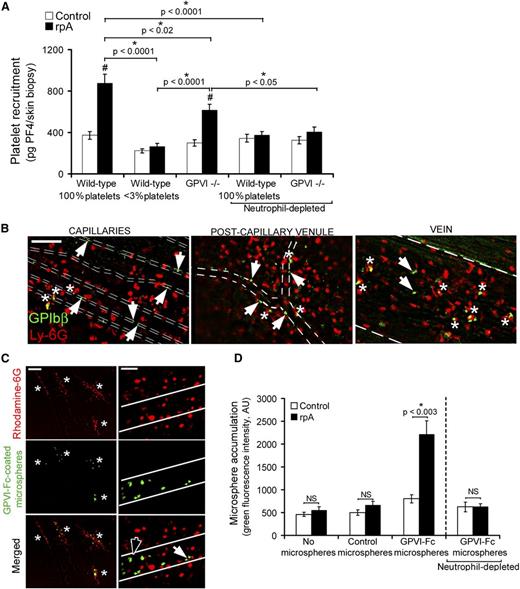
The fact that GPVI−/− mice displayed normal or even reduced neutrophil infiltration and degranulation, as well as reduced oxidative stress and preserved clearing of neutrophil elastase, suggested that their proneness to bleeding during IC-mediated inflammation was not linked to increased neutrophil aggressiveness or to a lack of platelet buffering action toward neutrophil toxic activities. An alternative possibility is that GPVI coordinates platelet-mediated vascular repair. In order to explore this hypothesis, we assessed whether platelets were actively recruited to the inflamed vasculature at the rpA reaction site and, if so, whether GPVI substantially contributed to this recruitment. Platelet recruitment was estimated by measuring platelet factor 4 (PF4), a protein abundantly stored in platelet α-granules, in skin biopsy specimens of control or rpA reaction areas. Importantly, to limit interferences due to passive deposition of platelets through bleeding, skin biopsy specimens were harvested 90 minutes after rpA reaction elicitation, before any significant bleeding was visible in GPVI−/− mice. In wild-type mice, the rpA reaction led to an increase in PF4 skin content that was dependent on platelets, as shown by its almost complete abrogation in mice rendered thrombocytopenic prior to the induction of the rpA reaction (Figure 5A). In GPVI−/− mice, the rpA reaction also led to an increase in PF4 skin content, but to a lesser extent than in wild-type mice (Figure 5A). Remarkably, platelet recruitment to the inflamed skin was inhibited in both wild-type and GPVI−/− mice that were depleted of neutrophils (Figure 5A). Together, these results show that neutrophils are responsible for the early recruitment of platelets at the reaction site and that GPVI contributes in part to this recruitment.
GPVI mediates vascular repair following neutrophil-induced injury. (A) Comparison of platelet accumulation at unstimulated control sites or at the site of the rpA reaction in the skin of control wild-type, thrombocytopenic, and GPVI−/− mice, as assessed by measurement of skin PF4 content. n = 6-15 mice per group. # indicates a significant difference (P < .0001) as compared with the PF4 content in unstimulated control skin of wild-type mice. (B) Platelets and neutrophils were stained in vivo using fluorescent antibodies to GPIbβ and Ly-6G, respectively, and their interactions with vessels during the rpA reaction were analyzed by intravital microscopy through a dorsal skinfold chamber. Whether at the capillary, postcapillary venule, or vein level, single platelets were seen binding directly to the vessel lining (white arrowheads) or to adherent neutrophils (white asterisks). Vessel edges are highlighted by dotted lines. The images shown were taken between 90 minutes and 2 hours after inducing the rpA reaction and are representative of 4 independent experiments. Scale bar, 100 µm. (C) The interactions of fluorescent microspheres (2 µm diameter) coated with a chimeric form of GPVI (GPVI-Fc) with the inflamed vasculature were analyzed by intravital microscopy. GPVI-Fc-coated microspheres accumulated specifically at sites of leukocyte recruitment and infiltration (white asterisks) identified by rhodamine-6G labeling. Observation of the GPVI-Fc–coated microspheres at higher magnification revealed individual microspheres binding directly to the vessel wall (black arrowhead) or to adherent leukocytes (white arrowhead). Vessel edges are highlighted in white. Scale bars, 200 µm (left) and 50 µm (right). The images shown are representative of 4 independent experiments. (D) Quantification of control and GPVI-Fc–coated microsphere accumulation at unstimulated control sites or at the site of the rpA reaction in the skin of wild-type mice that were immunodepleted or not for neutrophils, as indicated. n = 5-8 mice per group. NS, not significant.
GPVI mediates vascular repair following neutrophil-induced injury. (A) Comparison of platelet accumulation at unstimulated control sites or at the site of the rpA reaction in the skin of control wild-type, thrombocytopenic, and GPVI−/− mice, as assessed by measurement of skin PF4 content. n = 6-15 mice per group. # indicates a significant difference (P < .0001) as compared with the PF4 content in unstimulated control skin of wild-type mice. (B) Platelets and neutrophils were stained in vivo using fluorescent antibodies to GPIbβ and Ly-6G, respectively, and their interactions with vessels during the rpA reaction were analyzed by intravital microscopy through a dorsal skinfold chamber. Whether at the capillary, postcapillary venule, or vein level, single platelets were seen binding directly to the vessel lining (white arrowheads) or to adherent neutrophils (white asterisks). Vessel edges are highlighted by dotted lines. The images shown were taken between 90 minutes and 2 hours after inducing the rpA reaction and are representative of 4 independent experiments. Scale bar, 100 µm. (C) The interactions of fluorescent microspheres (2 µm diameter) coated with a chimeric form of GPVI (GPVI-Fc) with the inflamed vasculature were analyzed by intravital microscopy. GPVI-Fc-coated microspheres accumulated specifically at sites of leukocyte recruitment and infiltration (white asterisks) identified by rhodamine-6G labeling. Observation of the GPVI-Fc–coated microspheres at higher magnification revealed individual microspheres binding directly to the vessel wall (black arrowhead) or to adherent leukocytes (white arrowhead). Vessel edges are highlighted in white. Scale bars, 200 µm (left) and 50 µm (right). The images shown are representative of 4 independent experiments. (D) Quantification of control and GPVI-Fc–coated microsphere accumulation at unstimulated control sites or at the site of the rpA reaction in the skin of wild-type mice that were immunodepleted or not for neutrophils, as indicated. n = 5-8 mice per group. NS, not significant.
We then investigated the interactions between platelets and neutrophils during the cutaneous rpA reaction by intravital microscopy in mice bearing dorsal skinfold chambers and that were injected with fluorescent antibodies to platelets (anti-GPIbβ) and neutrophils (anti-Ly6G). Single platelets and neutrophils of wild-type mice started to adhere in venules and capillaries of the stimulated area within minutes of rpA reaction induction. After 2 hours of reaction, individual adherent platelets were still present in the inflamed vasculature, but no thrombi or platelet clumps had developed (Figure 5B). Whereas neutrophils were located in both the intracellular and extravascular compartments, platelets were found mostly inside vessels (Figure 5B). Adherent platelets in inflamed skin vessels included platelets interacting directly with the vessel lining and platelets sticking to adherent neutrophils (Figure 5B). These results suggest that early during the rpA reaction, neutrophils recruit platelets by binding them directly and also by unmasking platelet-binding sites on the vessel wall. Notably, in GPVI−/− mice, the residual platelets that were recruited to the reaction site also bound to the vessel wall and to adherent neutrophils (not shown), thus indicating that redundant mechanisms independent of GPVI exist to support both types of interactions.
To further determine whether the GPVI-dependent component of platelet recruitment contributed to the repair of neutrophil-inflicted vascular damage during the rpA reaction, we used fluorescent microspheres coated with a chimeric form of GPVI consisting of its extracellular domain fused to the Fc part of a human IgG1 molecule (GPVI-Fc)20 to detect exposure of GPVI-binding sites. The functionality of the GPVI-Fc–coated microspheres was evaluated in vitro by checking their increased ability to bind fibrillar collagen as compared with control microspheres coated with irrelevant rabbit IgG (supplemental Figure 2). Intravital microscopy analysis of GPVI-Fc–coated microspheres within the inflamed vasculature during the course of the cutaneous rpA reaction revealed interactions closely resembling those observed for platelets. In contrast to control microspheres that did not show any interactions (not shown), firmly adherent GPVI-Fc–coated microspheres interacting with the vessel wall alone or with recruited leukocytes were seen at the reaction site within vessels where leukocytes accumulated (Figure 5C). Quantification of control and GPVI-Fc–coated microspheres by measurement of green fluorescence in skin biopsy specimens confirmed that GPVI-Fc–coated microspheres accumulated locally during the rpA reaction (Figure 5D). These results show that the rpA reaction leads to the exposure of binding sites for GPVI on the vessel wall and on neutrophils themselves and suggest that GPVI alone is sufficient to support firm adhesion in this situation. Depletion of neutrophils prior to the induction of the rpA reaction completely abolished the accumulation of GPVI-Fc–coated microspheres at the reaction site (Figure 5D), indicating that in addition to directly binding GPVI, neutrophils are also fully responsible for the exposure of GPVI-binding sites on the inflamed vessel wall. Taken together, these results indicate that GPVI contributes to the prevention of bleeding in inflamed tissues by supporting platelet adhesion to sites of neutrophil-induced vessel injury.
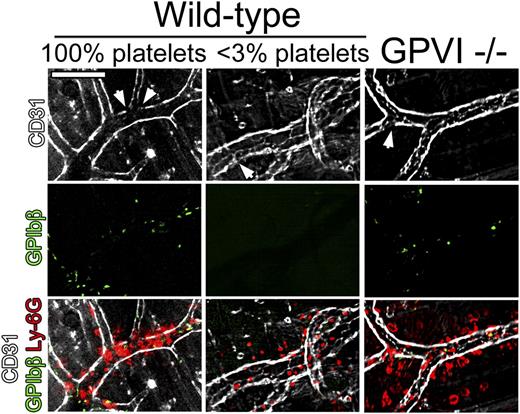
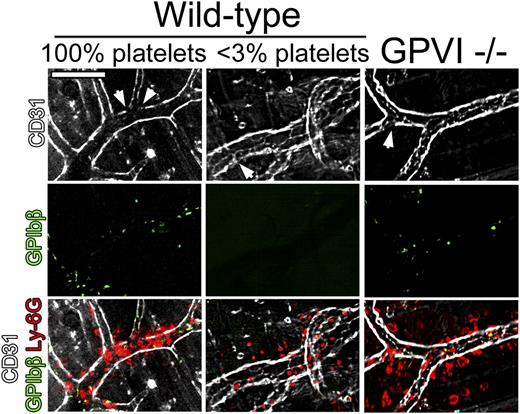
To assess the extent of endothelial damage at sites of neutrophil infiltration during the cutaneous rpA reaction in wild-type, GPVI−/−, and thrombocytopenic mice, in vivo staining of the endothelial lining using fluorescent antibodies to CD31 was performed. In all groups of mice, although CD31 staining of blood vessels was globally preserved, faint or absent CD31 staining was found in areas of neutrophil accumulation (Figure 6). Notably, preserved CD31 staining was also observed in some bleeding vessels in GPVI−/− and thrombocytopenic mice. These results suggest that limited damage to the endothelium and/or endothelial retraction is sufficient to cause bleeding in the absence of platelets or GPVI.
Neutrophil infiltration and endothelial integrity during the early phase of the cutaneous rpA reaction. The integrity of the endothelial cell layer at sites of neutrophil infiltration during the cutaneous rpA reaction was investigated by intravital microscopy. Endothelial cells, platelets, and neutrophils were stained using fluorescent antibodies to CD31, GPIbβ, and Ly-6G, respectively. In all groups of mice, although faint CD31 staining (arrowheads) was found in areas of neutrophil infiltration, the endothelial cell layer was overall well preserved. All pictures were taken during the first 3 hours of reaction. Scale bar, 100 µm.
Neutrophil infiltration and endothelial integrity during the early phase of the cutaneous rpA reaction. The integrity of the endothelial cell layer at sites of neutrophil infiltration during the cutaneous rpA reaction was investigated by intravital microscopy. Endothelial cells, platelets, and neutrophils were stained using fluorescent antibodies to CD31, GPIbβ, and Ly-6G, respectively. In all groups of mice, although faint CD31 staining (arrowheads) was found in areas of neutrophil infiltration, the endothelial cell layer was overall well preserved. All pictures were taken during the first 3 hours of reaction. Scale bar, 100 µm.
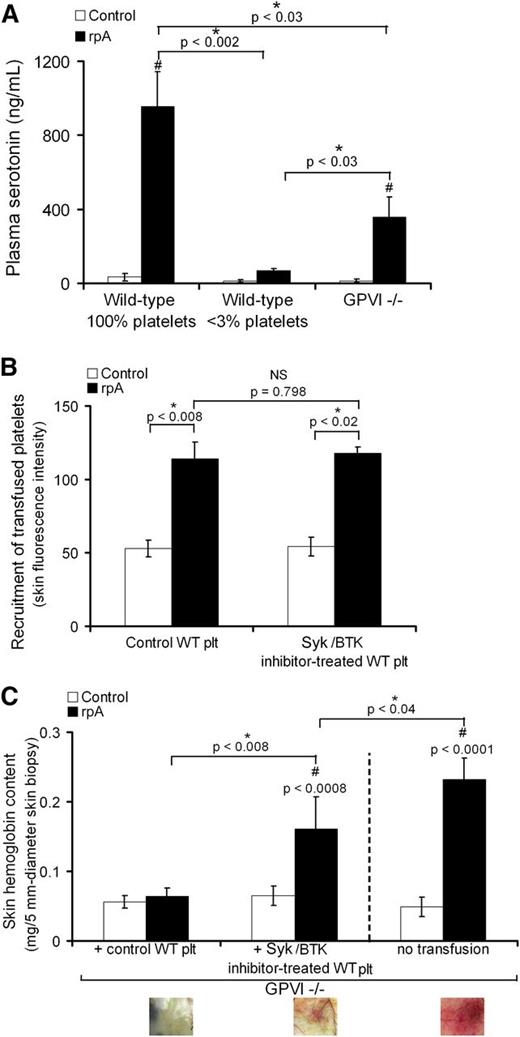
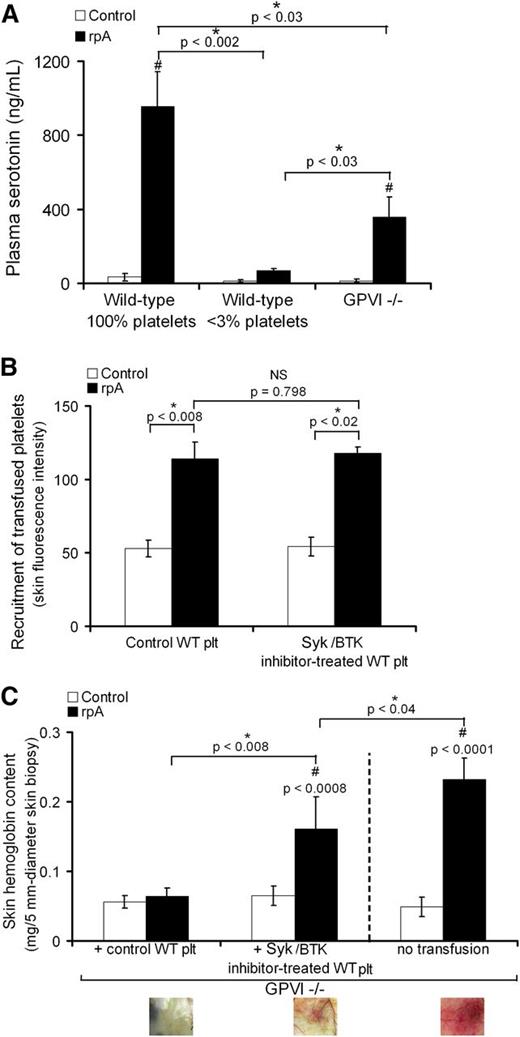
GPVI signaling supports platelet secretion and contributes to the vasculoprotective action of platelets during IC-mediated skin inflammation
Because in addition to being an adhesion receptor, GPVI is also a signaling receptor, we assessed the contribution of GPVI signaling to the prevention of bleeding by platelets during IC-mediated inflammation. We first determined whether the rpA reaction was associated with the release of platelet-derived soluble factors. For this analysis, we focused on the release of serotonin, a factor for which platelet-dense granules represent the main source in blood.21 In control wild-type mice with normal platelet counts, the cutaneous rpA reaction led to a marked increase in plasma serotonin (Figure 7A). This increase was fully prevented in mice rendered thrombocytopenic prior to the induction of the rpA reaction and partially in GPVI−/− mice (Figure 7A). These results show that the rpA reaction is accompanied by a platelet secretory response that is in part dependent on GPVI-mediated signaling. We then compared the ability of control wild-type platelets to rescue inflammatory skin bleeding in GPVI−/− mice to that of wild-type platelets that were treated with inhibitors of spleen tyrosine kinase (Syk) and the Bruton tyrosine kinase (Btk). Both Syk and Btk are protein tyrosine kinases crucial for ITAM-dependent signaling, notably downstream of GPVI.22-24 Before transfusion into GPVI−/− mice, the 2 types of washed platelets were loaded with calcein-AM to allow their tracking. Both control wild-type platelets and platelets treated with the Syk inhibitor R406 and the Btk inhibitor ibrutinib (Syk/BTK-inhibitor–treated platelets) accumulated at the site of the cutaneous rpA reaction, to a similar level (Figure 7B). However, whereas control wild-type platelets fully prevented inflammatory skin bleeding in GPVI−/− mice, the Syk/BTK-inhibitor–treated platelets only partially corrected the bleeding phenotype of GPVI−/− mice (Figure 7C). Overall, these results indicate that prevention of inflammatory bleeding by platelets requires both GPVI-dependent platelet adhesion and signaling.
GPVI-dependent signaling contributes to platelet secretion and prevention of bleeding during the cutaneous rpA reaction. (A) Plasma serotonin levels in control (100% platelets), thrombocytopenic (<3% platelets), and GPVI−/− mice that were subjected or not to the cutaneous rpA reaction. n = 5-8 mice per group. # indicates a significant difference (P < .002) as compared with plasma serotonin level in unchallenged control wild-type mice. (B) GPVI−/− mice were transfused with 9 × 106 green-fluorescent calcein-AM–labeled wild-type platelets that were treated or not with the Syk inhibitor R406 and the Btk inhibitor ibrutinib, as indicated. The cutaneous rpA reaction was then elicited, and the recruitment of transfused platelets to the reaction site was quantified by measurement of green fluorescence intensity in extracts from skin biopsy specimens harvested after 4 hours of reaction. (C) Hemoglobin content in skin biopsy specimens from control and rpA reaction areas from GPVI−/− mice and from GPVI−/− mice that were transfused with control or Syk/Btk-inhibitor–treated wild-type platelets. n = 4-8 mice per group. # indicates a statistically significant difference (P < .05) in hemoglobin content as compared with control spots from GPVI−/− mice transfused with control wild-type platelets. Representative pictures of the skin at the site of the rpA reaction are shown below. NS, not significant; WT, wild-type.
GPVI-dependent signaling contributes to platelet secretion and prevention of bleeding during the cutaneous rpA reaction. (A) Plasma serotonin levels in control (100% platelets), thrombocytopenic (<3% platelets), and GPVI−/− mice that were subjected or not to the cutaneous rpA reaction. n = 5-8 mice per group. # indicates a significant difference (P < .002) as compared with plasma serotonin level in unchallenged control wild-type mice. (B) GPVI−/− mice were transfused with 9 × 106 green-fluorescent calcein-AM–labeled wild-type platelets that were treated or not with the Syk inhibitor R406 and the Btk inhibitor ibrutinib, as indicated. The cutaneous rpA reaction was then elicited, and the recruitment of transfused platelets to the reaction site was quantified by measurement of green fluorescence intensity in extracts from skin biopsy specimens harvested after 4 hours of reaction. (C) Hemoglobin content in skin biopsy specimens from control and rpA reaction areas from GPVI−/− mice and from GPVI−/− mice that were transfused with control or Syk/Btk-inhibitor–treated wild-type platelets. n = 4-8 mice per group. # indicates a statistically significant difference (P < .05) in hemoglobin content as compared with control spots from GPVI−/− mice transfused with control wild-type platelets. Representative pictures of the skin at the site of the rpA reaction are shown below. NS, not significant; WT, wild-type.
Discussion
In line with previous studies pointing to a role of innate-immune cells in thrombocytopenia-associated bleeding in tumors, tumor necrosis factor-α–induced skin inflammation and glomerulonephritis,12,16 we show here that neutrophils are responsible for inflammatory bleeding in thrombocytopenic and GPVI−/− mice during IC-mediated inflammation. The fact that neutrophils cause inflammatory bleeding during thrombocytopenia sheds new light on results from previous studies addressing the vasculoprotective action of platelets during inflammation. When the role of GPVI in maintenance of vascular integrity during inflammation was initially investigated, FcRγ chain−/− mice were used as a model for GPVI deficiency. In contrast to the GPVI−/− mice we used here and to mice that were immunodepleted for GPVI,15 FcRγ chain−/− mice showed no abnormal susceptibility to inflammatory bleeding when subjected to IC-mediated skin inflammation.11 Although these data might appear contradictory at first sight, in the light of our results, this discrepancy can now be easily explained by the absolute requirement of Fcγ receptors for neutrophil extravasation during the rpA reaction25 and by the generally compromised innate immunity of mice lacking the FcRγ chain.26 Therefore, in a similar manner to FcRγ chain−/− mice, mice with a drug-induced or genetically induced platelet defect that would impair neutrophil infiltration could erroneously appear to be protected from inflammatory bleeding simply because neutrophil infiltration had not occurred in the first place.
The identification of neutrophils as the cause of inflammatory bleeding in thrombocytopenic mice led us to further explore how platelets manage to prevent their detrimental effects. We investigated the contribution of platelets to the regulation of neutrophil mobilization, infiltration, and degranulation and, ultimately, to the repair of neutrophil-inflicted vascular injury. In agreement with previous studies,27 our results show that platelets enhance neutrophil infiltration, as demonstrated by the increased neutrophil content at the rpA reaction site in mice with normal platelet counts as compared with thrombocytopenic mice, whether the reaction was induced in the skin or peritoneal cavity. Probably reflecting in part their decreased neutrophil infiltration, thrombocytopenic mice showed reduced levels of secreted MMP-9 and MPO and decreased oxidative stress in peritoneal lavage fluids after IC-induced peritonitis. The MPO degranulation index of thrombocytopenic mice was normal, indicating that platelets do not specifically regulate the release of primary granules in this setting. On the contrary, the degranulation index for MMP-9 was reduced in thrombocytopenic mice, thus indicating that platelets stimulate the secretion of neutrophil gelatinase granules. The normalization of the oxidative stress level with respect to neutrophil infiltration also showed that platelets stimulate oxidative activity during IC-mediated peritonitis. Although we cannot be certain that these observations made in IC-induced peritonitis also apply when the rpA reaction occurs in the skin, they are in line with the stimulating effect of platelets on neutrophil cytotoxic activities that was previously reported in other models of localized tissue inflammation.28,29
Regarding the role of the collagen receptor GPVI, GPVI−/− mice showed normal neutrophil infiltration when the rpA reaction was induced in the skin and a slightly lower, but nonsignificant, infiltration in comparison with controls when the reaction was elicited in the peritoneal cavity. Therefore, GPVI does not appear to play a major role in the enhancement of neutrophil infiltration by platelets at sites of IC-induced inflammation. However, GPVI−/− mice had reduced levels of secreted MMP-9 and a low degranulation index for MMP-9 that was similar to that of thrombocytopenic mice. It thus appears that GPVI is implicated in stimulation of neutrophil gelatinase granule secretion by platelets. The reduced oxidative index of GPVI−/− mice also suggests that GPVI contributes to the pro-oxidative action of platelets. Interestingly, a role for platelet GPVI in the regulation of MMP-9 production in innate immune cells was previously suggested by the results of a study showing that platelets stimulated MMP-9 production in monocytes only in the presence of collagen.30 Collectively, these results showing that platelets support neutrophil infiltration and degranulation indicate that prevention of neutrophil-dependent bleeding by platelets is not due to downregulation of neutrophil infiltration or degranulation. Furthermore, the implication of GPVI in the platelet-dependent enhancement of neutrophil secretion and oxidative activity during the rpA reaction corroborates the proinflammatory function of GPVI demonstrated in other inflammatory models.31
Our data revealed that another way by which platelets could counter neutrophil tissue-damaging activities was by exerting a buffering action via the scavenging of neutrophil elastase. An increase in platelet-associated neutrophil elastase indeed occurred during IC-mediated inflammation, suggesting that platelets scavenge neutrophil elastase from the reaction site and convey it into the circulation. However, the fact that GPVI−/− mice displayed inflammatory bleeding despite normal elastase scavenging suggests that elastase uptake by platelets is not essential or sufficient to inhibit or stop inflammatory bleeding.
Finally, we found that platelets were actively recruited to the inflamed vasculature where they adhered as single platelets bound to adherent neutrophils or directly to the vessel lining. However, during the 4 hours of the rpA reaction, no thrombus was detected. This observation showing that platelets can exert hemostatic functions independently of thrombus formation is in agreement with previous observations made in models of kidney and skin inflammation,11,12 and in solid tumors.32 Our study further shows that this recruitment of platelets relies in part on GPVI and is largely dependent on neutrophils, as indicated by the decreased platelet content in the inflamed skin of GPVI−/− mice compared with their wild-type littermates and by the complete prevention of platelet recruitment in neutropenic mice. A similar implication of neutrophils and GPVI in platelet recruitment to the inflamed vasculature was previously shown in a model of glomerulonephritis induced by IV injection of an antibody to the glomerular basement membrane.33 Consistent with our results, this previous study showed that neutrophil depletion and GPVI deficiency also reduced platelet accumulation in the inflamed glomerulus. Additionally, our intravital microscopy analysis revealed that neutrophils not only bind platelets directly but also unmask platelet- and GPVI-binding sites on the inflamed vessel wall. Our results therefore support the concept that the recruitment of platelets and neutrophils to sites of inflamed and/or injured vessels relies on 2-way cooperative interactions.33,34 In this context, it is interesting to note that the extracellular matrix metalloproteinase inducer (EMMPRIN) was recently identified as a leukocyte counterreceptor for GPVI expressed by neutrophils.35,36
When addressing the extent of endothelial damage during the early phase of the rpA reaction, we observed that the endothelial lining was generally preserved but displayed alterations in regions of neutrophil infiltration, thus suggesting that endothelial injury and/or endothelial retraction contribute to inflammatory bleeding. Surprisingly, whether in GPVI−/− or thrombocytopenic mice, not all vessels supporting neutrophil infiltration bled. This latter observation shows that for inflammatory bleeding to occur, neutrophil infiltration per se is not sufficient. Thus, additional factors such as the vessel condition and/or local concentration of neutrophil injurious substances are also likely to be involved. This could further explain why neutrophil infiltration did not cause bleeding when the rpA reaction was elicited in the peritoneal cavity of thrombocytopenic mice.
Given that GPVI is the main platelet receptor for collagen, it is likely that single platelets stop bleeding by covering small areas where the basement membrane gets exposed and disrupted by neutrophils. Nevertheless, although single adherent platelets could have exerted a purely mechanical action by plugging small holes in the endothelial lining, we show that platelets release soluble factors during the course of the rpA reaction and that prevention of platelet ITAM signaling partially impairs the ability of wild-type platelets to rescue inflammatory bleeding when transfused into GPVI−/− mice. It then appears that like in tumors,32 activation-dependent secretion and/or spreading of individual platelets bound to the vessel wall or to neutrophils also contribute to sealing of the vascular breaches inflicted by neutrophils. In that regard, we have previously shown that even when not causing aggregation, collagen binding to GPVI leads to the release of soluble platelet factors such as angiopoietin-1, serotonin, and adenosine triphosphate, all of which are regulators of endothelial cell barrier function.37 Moreover, platelets lacking the adapter protein SLP-76, which mediates GPVI-dependent platelet responses to collagen including degranulation and spreading, were previously shown to be unable to prevent inflammatory bleeding in the very same model of cutaneous rpA reaction we used here.15
In conclusion, our results demonstrate that although platelets exert proinflammatory functions by enhancing neutrophil infiltration, degranulation, and oxidative stress during IC-induced inflammation, they also control the associated risk of bleeding by sealing neutrophil-induced vascular breaches via GPVI-dependent platelet activation and binding to the injured vessel wall. More generally, our study suggests that platelets should be considered as integral players of IC-mediated inflammation that intervene not only to amplify the recruitment of neutrophils but also to regulate their toxic activities and repair the damage they induce. Finally, our results indicate that inflammation represents an unconventional hemostatic situation in which early adhesion and activation of individual platelets is sufficient to prevent bleeding.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Mary Osborne-Pellegrin for her help in editing this manuscript.
This work was supported by the Institut National de la Santé et de la Recherche Médicale and by grants from La Fondation de France (2014/GRE/LL/HB/205-U1148), DHU FIRE, CORDDIM Région Ile de France (B.H.-T.-N.), and the Deutsche Forschungsgemeinschaft (GO1360/4-1) (T.G.).
Authorship
Contribution: A.G., L.L., V.O., V.S., S.L., and B.H.-T.-N. designed and performed experiments and analyzed data; T.G, B.N., and M.J.-P. provided reagents and conceptual advice; B.H.-T.-N. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Benoît Ho-Tin-Noé, INSERM U1148, Hôpital Bichat, 46 rue Henri Huchard, 75877 Paris Cedex 18, France; e-mail: benoit.ho-tin-noe@inserm.fr.