Key Points
The risk of transformation of NLPHL to DLBCL is 0.74 per 100 patient-years of follow-up.
Risk factors for transformation include prior exposure to chemotherapy and splenic involvement at time of diagnosis.
Abstract
A number of reports have shown a propensity of nodular lymphocyte–predominant Hodgkin lymphoma (NLPHL) to transform into diffuse large B-cell lymphoma (DLBCL). Long-term data on the incidence and outcomes of transformed NLPHL are lacking. A comprehensive analysis of the actively maintained Mayo Clinic Lymphoma Database was performed. Between 1970 and 2011, 222 consecutive adult patients with new untreated NLPHL were identified. Median age at diagnosis was 40 years, and 146 (66%) were males. The median follow-up was 16 years. Seventeen patients (7.6%) developed a transformation to DLBCL. The median time to transformation was 35 months (range, 6-268 months). Based on the observed 17 transformations during 2304 patient-years of follow-up, the rate of transformation was 0.74 per 100 patient-years. In a multivariate analysis, use of any prior chemotherapy (P = .04) and splenic involvement (P = .03) were significantly associated with increased risk of transformation. The 5-year overall survival (OS) in those with transformed disease was 76.4%, and transformation did not adversely affect OS when compared with patients who did not experience transformation. In this large single-institution cohort with long-term follow-up, the risk of transformation was lower than that observed in other low-grade lymphomas.
Medscape Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint providership of Medscape, LLC and the American Society of Hematology.
Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 2043.
Disclosures
Laurie Sehn, Associate Editor, served as an advisor or consultant for Amgen, Genentech, Gilead, Janssen, Lundbeck, Roche, and Seattle Genetics. Laurie Barclay, freelance writer and reviewer, Medscape, LLC, owns stock, stock options, or bonds from Pfizer. The authors declare no competing financial interests.
Learning objectives
Identify the incidence of transformation of nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) to diffuse large B-cell lymphoma (DLBCL), based on an analysis of the Mayo Clinic Lymphoma Database.
Evaluate risk factors for transformation of NLPHL to DLBCL.
Determine outcomes of transformation of NLPHL to DLBCL.
Release date: April 21, 2016; Expiration date: April 21, 2017
Introduction
Nodular lymphocyte–predominant Hodgkin lymphoma (NLPHL) is an uncommon subtype of Hodgkin lymphoma (HL), comprising 3% to 5% of HL patients. Due to its unique clinical, histologic, and immunophenotypical features, it is recognized as a distinct entity in the World Health Organization (WHO) classification.1,2 NLPHL is characterized by proliferation of neoplastic lymphocyte predominant cells (formerly known as lymphocytic and histiocytic cells or Reed-Sternberg [RS] variant) rather than RS cells seen in classical HL.1 These cells exhibit B-cell markers, including CD20, CD19, CD79, immunoglobulin gene rearrangements, and epithelial membrane antigen, but lack expression of CD30 and CD15, in contrast to classical HL, where RS cells typically express CD15, CD30 and lack expression of B-cell markers.3
Clinically, the disease predominately affects male patients in the third or fourth decade of life, and it tends to have an indolent course, in most cases similar to other low-grade non-HLs, with a tendency for late relapses.4-7 Most patients present with localized disease, and the findings of extranodal disease, bone marrow involvement, or B symptoms are not common. NLPHL is associated with a favorable overall prognosis.4-7 Histopathological variants of NLPHL are associated with increased relapse and advanced stages.8
Prior observations have reported an increased risk of transformation to aggressive lymphoma, which has been reported to be higher than what is observed in classical HL.4,9-15 Transformation can be seen concurrently or subsequent to the time of NLPHL diagnosis.11 The incidence of transformation has varied in different studies. Aggressive lymphomas arising from NLPHL were found to have heterogeneous morphology and immunophenotype.16 Most studies have shown that transformation is associated with significantly inferior outcomes.4,9-14
Due to the B-cell phenotype of lymphocyte-predominant cells, a clonal correlation has been postulated to exist between NLPHL and aggressive lymphomas. Small studies have examined this correlation by sequencing the involved immunoglobulin gene region and reported shared clonality between the two disease processes.17-19
The variability in reported incidence and outcomes of histologic transformation may reflect the rarity of the disease (small sample size), inadequate follow-up, lack of pathological confirmation at time of transformation, and histological heterogeneity of transformed aggressive lymphomas. Risk factors for transformation are not known, although one study suggested that splenic involvement at time of NLPHL diagnosis might represent a risk factor for future transformation.20
The goals of this study were to assess incidence, outcomes, risk factors for and long-term follow-up of transformation of NLPHL to diffuse large B-cell lymphoma (DLBCL) over a 40 year-experience from a single institution.
Methods
Patients and materials
The actively maintained prospective Mayo Clinic Lymphoma Database includes patients consenting to have their medical records reviewed for research. All consecutive consenting patients with lymphoma seen at the Mayo Clinic Rochester and diagnosed with NLPHL between 1970 and 2011 were included in this study. The study was approved by the institutional review board. The initial screen identified 235 patients with NLPHL. Of these, 13 were pediatric patients (younger than 13 years) and were excluded from this analysis. Pathology from initial diagnosis was reviewed by the study hematopathologist (W.R.M.) according to WHO criteria.1,2
A total of 222 patients were included in this study. The clinical characteristics, including age, sex, stage at presentation, B symptoms, splenic involvement, prior therapies, and treatments at time of transformation, were recorded and analyzed. Limited stage included stage I and II disease, and advanced stage included stage III and IV disease. Splenic involvement was diagnosed by imaging, pathology, or both. Since the study spanned over 4 decades, different chemotherapeutic regimens were used. These included ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine), CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), MOPP (mustargen, oncovin, procarbazine, and prednisone), R-CHOP (rituximab-CHOP), R-ABVD, MOPP/ABVD, BCVPP (BCNU, cyclophosphamide, vincristine, prednisone, and procarbazine), CVP (cyclophosphamide, vincristine, and prednisone), and ProMACE-CytaBOM (prednisone, vincristine, methotrexate, doxorubicin, cyclophosphamide, etoposide, cytarabine, bleomycin, and leucovorin).
Transformation to diffuse large B-cell lymphoma
The diagnosis of histologic transformation was based on a biopsy confirmation in accordance with the WHO criteria for DLBCL.1,2 Patients were included only when histologic transformation followed the diagnosis of NLPHL.
The diagnosis of other types of aggressive lymphomas (other than DLBCL) was rare. This included 1 case (0.5%) of peripheral T-cell lymphoma, not otherwise specified. In order to accurately assess rate and risk factors for transformation to DLBCL, patients who developed other types of lymphoma (n = 1) were not included in transformation and risk factor analyses. Patients diagnosed concurrently with a combination of NLPHL and DLBCL (composite lymphoma, 2 patients), and patients in whom the diagnosis of DLBCL preceded the diagnosis of NLPHL (2 patients) were also excluded from transformation risk analyses.
Definitions and statistical analysis
Freedom from transformation was measured from the time of diagnosis of NLPHL to time of diagnosis of DLBCL. Patients without transformation were censored at time of death or last follow up. The overall survival (OS) was calculated from the time of diagnosis of NLPHL (or from time of transformation to DLBCL) to the date of death or date of last follow-up, as indicated. In transformed patients, disease-free survival (DFS) was measured from the date of transformation to the date of relapse of either NLPHL or DLBCL. Transformation rates were calculated using a person-years approach, in which number of documented transformations was divided by total person-years of follow-up. Rates were expressed as number of transformation per 100 person-years. Categorical and continuous variables were compared using the χ2 test and Student t test, respectively. The Kaplan-Meier method was used to calculate OS and freedom from transformation, and survival was compared using the log-rank test. Analysis of risk factors for transformation was performed using a univariate analysis. Multivariate analysis was performed using the Cox proportional hazards model that included only factors with a P value of <0.1 from the univariate model. A 2-sided P value of <.05 was considered to be statistically significant. All statistical analyses were performed using JMP version 9.0.1 statistical software (SAS Institute).
Results
Presentation and initial treatment of NLPHL patients
A total of 222 adult patients had confirmed NLPHL at initial diagnosis and were included. The majority were male (146, 66%), and 166 patients (75%) presented with limited-stage disease. The median age at diagnosis was 40 years (range, 15-81 years). The median follow-up of the entire cohort was 16.3 years (range, 1-42.8 years), and follow-up was complete (until death or transformation) in 190 (85.6%) patients. Table 1 summarizes baseline characteristics and treatments received at the time of initial diagnosis of NLPHL.
The most common treatment in limited-stage disease (stage I or II, 166 patients) was single-modality radiation therapy (RT), which was the treatment in 116 (70%) patients, while the most commonly used treatments in advanced-stage disease (stage III or IV, 56 patients), was chemotherapy (35 patients, 63%). ABVD was the most frequently used regimen (primary regimen in 24 patients and in combination with RT in 18 patients, incorporated in a total of 46% of all patients treated with chemotherapy). In total, 10/222 (4.5%) patients received rituximab as part of the initial treatment.
Eight patients (4%) were observed as initial management: 2 patients were diagnosed during pregnancy and had a brief period of observation until delivery, 1 patient was not a candidate for chemotherapy (died of unrelated causes after 27 months), 3 were observed after resection of a stage IA disease and had no further therapy (all died of unrelated causes after 5, 7.9, and 15.7 years of follow-up), 1 patient remains disease-free at 15 years, and 1 patient had progression of NLPHL after 11 years and was subsequently treated with MOPP chemotherapy.
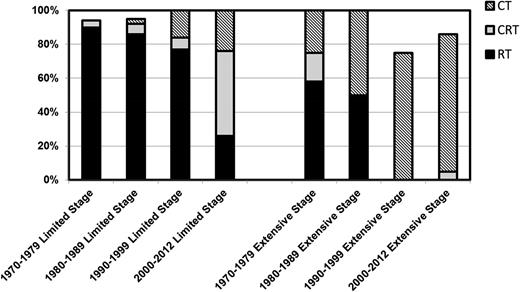
Since the study spanned over 4 decades, the approach to patients with limited and extended disease significantly changed over time (Figure 1). Consistent with changes of therapy for classical HL and non-HL, and the emergent role for chemotherapy combinations in HL and non-HL, such as R-CHOP21 and ABVD,22 there was a significant decline in the use of RT with time, particularly in limited-stage disease.
Changes in initial management strategies of NLPHL across 4 decades in 1 institution. CT, chemotherapy; CRT, chemoradiation therapy.
Changes in initial management strategies of NLPHL across 4 decades in 1 institution. CT, chemotherapy; CRT, chemoradiation therapy.
Incidence and risk of transformation to diffuse large B-cell lymphoma
Of the 222 patients in this cohort, 47 (21.2%) developed 1 or more relapses of NLPHL without a transformation to DLBCL. Two patients had a synchronous diagnosis of NLPHL and DLBCL, and in 2 patients, the diagnosis of DLBCL preceded the diagnosis of NLPHL. The latter 4 patients were excluded from transformation risk analyses
In addition, 5 patients were suspected to be relapsed based on their clinical presentation, positron emission tomography scan findings, and/or elevated lactate dehydrogenase levels. Of these, biopsy showed benign pathology in 4 patients (fibrosis in 1 patient, and follicular hyperplasia in 3 patients), and T-cell lymphoma (peripheral T-cell lymphoma, not otherwise specified) in 1 patient.
Eighteen (8.1%) of the 222 patients with confirmed NLPHL at diagnosis had a subsequent transformation to an aggressive lymphoma, and 17 had a confirmed transformation to DLBCL. The incidence of DLBCL transformation in NLPHL in our cohort was 7.6% and not significantly different across the years of treatment (7.4% for cases treated between 1970 and 1979, 8.1% for patients treated between 1980 and 1989, 5.8% for patients treated between 1990 and 1999, and 10.4% for patients treated between 2000 and 2012). In 3 patients (17.6%) with transformed disease, the transformation was in a site of prior radiation therapy, and in 3 patients (17.6%), the pathology was T-cell–rich B-cell lymphoma.
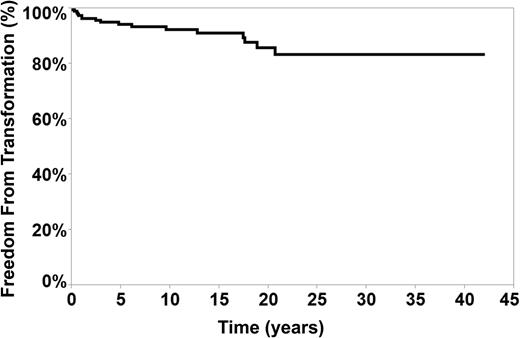
The median time to transformation, measured from the time of diagnosis of NLPHL was 35 months (range, 6-268 months). Figure 2 represents freedom from transformation measured from the time of diagnosis to the time of transformation. The rate of 40-year freedom from transformation was 83.4% in our cohort.
Using a person-year approach and based on the observed 17 transformations during the 16 years (2304 patient-years) of follow-up, the rate of transformation was 0.74 per 100 patient-years.
Characteristics, clinical presentation, and treatments of transformation to DLBCL
The median age at the time of transformation to DLBCL was 45 years (range, 30-87 years). Eleven of the 17 patients (65%) with transformed DLBCL presented with signs or symptoms at time of transformation (lymphadenopathy in 9 and B symptoms in 2 patients). Ten patients (59%) presented with advanced stage (III or IV), and most had high-risk disease based on the International Prognostic Index23 (score of 0 in 3 [18%] patients, 1 in 3 [18%] patients, 2 in 4 [23%] patients, and ≥3 in 7 [41%] patients). Three patients (18%) had bone marrow involvement, and 6 (35%) had extranodal diseases at the time of transformation.
The most frequently used regimens at the time of transformation were R-CHOP or CHOP in 9 patients (53%) (8 patients received R-CHOP and 1 received CHOP). Platinum-based therapy was incorporated in 4 (24%) patients (3 patients received R-ICE (rituximab, ifosfamide, carboplatin, and etoposide) and 1 received R-DHAP (rituximab, dexamethasone, high-dose Ara-C, cisplatin) because of prior exposure to anthracyclines); 2 of these patients then proceeded to consolidation autologous stem cell transplantation. Two patients (12%) received MOPP/ABVD; 1 (6%) was treated with bendamustine/rituximab and 1 (6%) was treated with single-agent rituximab. One patient received (6%) RT combined with R-CHOP for early-stage DLBCL, and 1 patient received intrathecal methotrexate in addition to R-CHOP for central nervous system involvement.
Outcomes following transformation to DLBCL
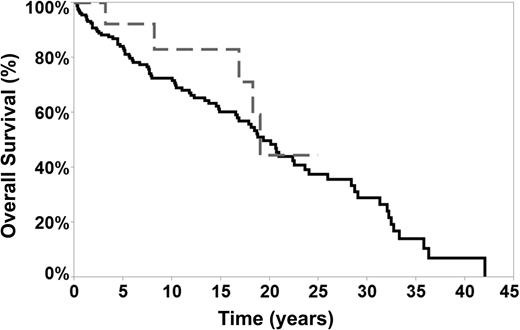
Sixteen of the 17 patients (94%) with a transformation to DLBCL achieved a complete remission after treatment. Four patients (24%) subsequently relapsed (DLBCL relapse in 1 patient and NLPHL relapse in 3 patients). All relapses were histologically confirmed with a biopsy. The median DFS measured from time of transformation was 87 (2-146) months, and the 5-year OS after transformation was 76% (4 patients [24%] had died, 3 of lymphoma and 1 of unrelated causes). The median OS, measured from the time of transformation was 8 years, and the median survival calculated from the time NLPHL diagnosis was 19 years. There was no statistically significant difference in OS between transformed or nontransformed NLPHL patients (Figure 3), indicating that transformation to DLBCL did not adversely affect OS. Clinical characteristics at presentation (age, sex, stage, splenic involvement, and B symptoms) and prior therapies (chemotherapy, RT, and anthracycline exposure) did not impact survival after transformation. However, due to the small number of transformed patients (17 patients), such comparisons are underpowered.
OS of transformed and nontransformed patients. The median OS in transformed patients was 18.8 years (dotted gray line) vs 19 years in nontransformed patients (black solid line).
OS of transformed and nontransformed patients. The median OS in transformed patients was 18.8 years (dotted gray line) vs 19 years in nontransformed patients (black solid line).
Since this study included patients with a median follow-up of 16 years (including 25% with more than 21 years of follow-up), 88 patients (40%) had died at the time of this analysis. The majority of deaths were of unrelated causes.
Clinical characteristics, initial treatments of NLPHL in transformed patients, and risk factors for transformation to DLBCL
In the 17 patients with transformed disease, the median age at diagnosis of NLPHL was 41.5 years (26-77). Eleven patients (65%) presented with limited stage (stage I or II), while 6 (35%) had advanced stage (III or IV) disease. Three patients (17.6%) had 1 relapse, and 2 patients (12%) had 2 or more relapses of NLPHL prior to transformation to DLBCL. Table 2 compares disease characteristics and prior treatments in transformed vs nontransformed patients. Transformed patients had significantly higher incidence of splenic involvement at the time of presentation of NLPHL (29% vs 5%, P = .003) and a trend toward more exposure to any prior chemotherapy (65% vs 43%, P = .09) compared with nontransformed patients.
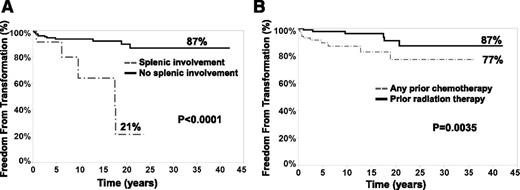
In a univariate analysis, splenic involvement at the time of NLPHL (P < .0001) diagnosis and prior exposure to any chemotherapy (chemotherapy or chemoradiation, as compared with patients treated with single-modality radiation therapy, P = .0035) were associated with a significantly shortened period of freedom from transformation (Table 3 and Figure 4). The 40-year rates of freedom from transformation were 87% if there was no splenic involvement vs 21% if there was splenic involvement and 87% for prior radiation therapy only vs 77% if there was a prior exposure to chemotherapy (Figure 4). B symptoms, sex, age, stage at presentation, and different chemotherapeutic regimens were not prognostic risk factors for transformation.
Splenic involvement (A) and prior exposure to chemotherapy (B) correlated to freedom from transformation to DLBCL in patients with NLPHL (222 patients). The rates of 40-year freedom from transformation were 87% when there was no splenic involvement (black solid line, A) vs 21% when the spleen was involved (dotted gray line, A) and 87% if radiation therapy was used as a single modality (black solid line, B) compared with 77% in patients treated with prior chemotherapy or chemoradiation combination (dotted gray line, B). Splenic involvement was diagnosed based on pathology or imaging studies.
Splenic involvement (A) and prior exposure to chemotherapy (B) correlated to freedom from transformation to DLBCL in patients with NLPHL (222 patients). The rates of 40-year freedom from transformation were 87% when there was no splenic involvement (black solid line, A) vs 21% when the spleen was involved (dotted gray line, A) and 87% if radiation therapy was used as a single modality (black solid line, B) compared with 77% in patients treated with prior chemotherapy or chemoradiation combination (dotted gray line, B). Splenic involvement was diagnosed based on pathology or imaging studies.
On a multivariate analysis, both exposure to chemotherapy and prior splenic involvement were predictive of a shortened period of freedom from transformation (hazard ratio, 4.3; 95% confidence interval, 1.7-10.5 [P = .04] and hazard ratio, 5.5; 95% CI, 2.0-12.5 [P = .03], respectively)
Discussion
To our knowledge, this cohort represents the largest analysis to date of consecutive patients with NLPHL with long-term follow-up. Importantly, most patients were entered into the Mayo Clinic Lymphoma Database at the time of initial diagnosis of untreated NLPHL and prospectively followed. In this cohort, the incidence of transformation to DLBCL was 7.6%, with an annual risk of 0.74 per 100 patient-years of follow up. Transformation did not adversely affect OS. This analysis suggests that prior exposure to chemotherapy and a presentation with splenic involvement are associated with increased risks of transformation.
The tendency of NLPHL to transform to aggressive lymphomas has been well recognized, and the incidence of such a transformation has been reported to range from 2% to 17% in different studies.4,9-11,13-15,18,20,24 This wide range is likely due to small sample size,10,14,24 short duration of follow-up,20,24 lack of pathological confirmation of transformation,20 inclusion of patients diagnosed with concurrent and subsequent DLBCL in the same study,11 and analyzing the risk of transformation in all types of HL.9 Some studies have reported a significantly inferior survival in transformed patients compared with nontransformed patients.13,14,20 An analysis from the University of Nebraska group showed that the OS of these patients was similar to age- and sex-matched patients with de novo DLBCL.11
Our cohort of 222 patients had the classic features of NLPHL described in literature.4-7 Most were men who presented with limited-stage disease and B symptoms; bone marrow involvement or extranodal disease was not common. The median OS in our cohort was reached at 19 years. The median survival is shorter than what has been reported in other studies,13,20 likely related to the significantly longer period of follow-up, ie, reflecting actual vs estimated survival. Since our study spanned over 4 decades, 88 of these 204 patients had not survived at the time of this analysis, which is the main reason that our median survival was reached.
In total, 17 patients had DLBCL that developed after NLPHL diagnosis and were therefore included in our analysis. Four patients had concurrent (2 patients) or preceding DLBCL (2 patients). Including these 4 patients in the study would falsely increase the incidence of transformation. In addition, there was a high clinical suspicion for transformation in 5 patients, but biopsy of the FDG-avid lymph node demonstrated benign pathology or NLPHL. This emphasizes the need for a biopsy confirmation prior to establishing the diagnosis of histologic transformation, since this has major implications for subsequent therapy.
At the time of transformation, most patients presented with advanced-stage disease with adverse features, a finding that is consistent with previous analyses of DLBCL in patients with NLPHL.13,20 All patients were treated with curative intent, and the overall response rate to chemotherapy was 94%. The median DFS after transformation was 87 months. The median OS was 18.8 years. Transformed patients had an OS similar to nontransformed patients (Figure 3). The OS after transformation to DLBCL is superior to reported outcomes after transformed follicular lymphoma25 or clonally related transformed chronic lymphocytic leukemia (CLL), suggesting a different biology of transformation.
Outcomes after transformation to DLBCL in this cohort were much improved compared with other studies of transformed NLPHL.11,13,20 A potential explanation for this difference is the wide use of rituximab. Rituximab was incorporated in 82% of patients with transformed NLPHL in our study compared with 46% in the British Columbia Cancer Agency study20 and 5% in the Nebraska group study.11 The incorporation of rituximab in the initial management of follicular lymphoma has been reported to decrease the incidence of transformation.26
Finally, our prognostic model identified risk factors for transformation to DLBCL. Splenic involvement at the time of NLPHL diagnosis and prior exposure to chemotherapy were associated with higher risk of transformation (Figure 4). The finding of splenic involvement as a risk factor for transformation was reported by previous investigators.20 Interestingly, the association between exposure to prior chemotherapy and reduced freedom from transformation has not been reported in the past, but it has been observed in other low-grade lymphoma studies. In follicular lymphoma25 and CLL,27 heavily pretreated patients, especially those pretreated with purine analogs, had an increased risk of transformation to aggressive lymphomas compared with patients placed on observation.27,28 Additionally, analysis by Chen and colleagues showed that the use of chemotherapy without radiation in early-stage NLPHL was associated with early disease progression.29
The mechanism of how chemotherapy is associated with a reduced freedom from transformation is unclear, but it could be related to the fact that patients deemed to have higher-risk disease by the treating physicians (independent of stage or B symptoms) were recommended chemotherapy over radiation therapy only.
In summary, this large study of patients with NLPHL with long-term follow-up demonstrated that the risk of transformation to DLBCL was 0.74 per 100 patient-years of follow up, lower than what has been reported with transformed follicular lymphoma or CLL. In contrast to FL, transformed NLPHL is not associated with an adverse impact on OS, suggesting a possibly different biology of transformation. Presentation with splenic involvement and prior exposure to any chemotherapy were identified as risk factors to transformation and were associated with significantly shorter freedom from transformation in this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The study was supported in part by Lymphoma SPORE CA97274-12 and the Predolin Foundation.
Authorship
Contribution: S.S.K. and G.S.N. designed the study concept, performed research, analyzed data and wrote the manuscript; K.M.R. collected data and maintained the database; T.M.H., W.R.M., S.M.A., J.P.C., P.B.J., D.J.I., S.N.M., I.N.M., C.A.T., L.F.P., J.A.M., and T.E.W. participated in designing research; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Grzegorz S. Nowakowski, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: nowakowski.grzegorz@mayo.edu.