Key Points
SPRY2 is downregulated in CLL cells from patients with poor prognosis.
SPRY2 is negative regulator of Syk-mediated BCR and MAPK-Erk signaling in CLL.
Abstract
Clinical heterogeneity is a major barrier to effective treatment of chronic lymphocytic leukemia (CLL). Emerging evidence suggests that constitutive activation of various signaling pathways like mitogen-activated protein kinase–extracellular signal-regulated kinase (MAPK-Erk) signaling plays a role in the heterogeneous clinical outcome of CLL patients. In this study, we have investigated the role of Sprouty (SPRY)2 as a negative regulator of receptor and nonreceptor tyrosine kinase signaling in the pathogenesis of CLL. We show that SPRY2 expression is significantly decreased in CLL cells, particularly from poor-prognosis patients compared with those from good-prognosis patients. Overexpression of SPRY2 in CLL cells from poor-prognosis patients increased their apoptosis. Conversely, downregulation of SPRY2 in CLL cells from good-prognosis patients resulted in increased proliferation. Furthermore, CLL cells with low SPRY2 expression grew more rapidly in a xenograft model of CLL. Strikingly, B-cell–specific transgenic overexpression of spry2 in mice led to a decrease in the frequency of B1 cells, the precursors of CLL cells in rodents. Mechanistically, we show that SPRY2 attenuates the B-cell receptor (BCR) and MAPK-Erk signaling by binding to and antagonizing the activities of RAF1, BRAF, and spleen tyrosine kinase (SYK) in normal B cells and CLL cells. We also show that SPRY2 is targeted by microRNA-21, which in turn leads to increased activity of Syk and Erk in CLL cells. Taken together, these results establish SPRY2 as a critical negative regulator of BCR-mediated MAPK-Erk signaling in CLL, thereby providing one of the molecular mechanisms to explain the clinical heterogeneity of CLL.
Introduction
Chronic lymphocytic leukemia (CLL) is a clinically heterogeneous B-cell neoplasm that represents the most common form of adult leukemia in the United States.1 Based on the immunoglobulin variable heavy chain (IgVH) mutational status, chromosomal abnormalities, and cell surface markers, CLL patients are categorized into good- or poor-prognosis groups. Recent studies have identified a small actively proliferating population of CLL cells that reside in micro-anatomical sites known as proliferation centers (PCs).2 CLL cells receive diverse stimuli promoting their proliferation and survival in these PCs.3-5 We have previously used Gene Expression Profiling to decipher the diverse signaling that regulates the survival and proliferation of CLL cells in PCs. These studies revealed a critical role for B-cell–receptor (BCR) and mitogen-activated protein kinase–extracellular signal-regulated kinase (MAPK-Erk) signaling in the survival and proliferation of CLL cells.5 Furthermore, Gardener et al have recently reported that 36% of CLL patients possess mutations associated with activation of MAPK-Erk signaling pathways.6 Similarly, BCR signaling is upregulated in CLL, providing a chronic stimulus for their proliferation.3-5
Precise regulation of cellular processes, such as those mediated by B cells, requires homeostatic integration between intrinsic and extrinsic factors.7,8 Deregulation of such homeostatic mechanisms in CLL cells can lead to aberrant activation of MAPK-Erk and BCR signaling. Constitutive activation of BCR and MAPK-Erk signaling promotes CLL cell survival and proliferation.9-14 However, the molecular mechanisms that lead to the constitutive activation of these pathways have not been fully explored. Identifying novel regulators of these pathways in CLL is crucial for understanding the disease biology and for the eventual development of targeted therapies.
To identify potential regulators of BCR and MAPK-Erk signalingin CLL, we performed a transcriptome analysis for genes that are differentially expressed in CLL patients with good vs poor prognosis. Of interest in relationship to MAPK-Erk signaling, we observed that expression of Sprouty (SPRY)2, a member of the SPRY protein family, to be significantly downregulated in CLL cells from poor-prognosis patients compared with those from good-prognosis patients. SPRY proteins play key roles in maintaining cellular homeostasis by attenuating signaling, downstream to several ligand-induced receptor tyrosine kinases (RTKs).7-10 Hence, we reasoned that SPRY2 might act as a negative regulator of BCR signaling to inhibit the survival and proliferation of CLL cells. Therefore, we hypothesized that low levels of SPRY2 lead to a state of constitutive activation of BCR and MAPK-Erk signaling in poor-prognosis CLL patients. Consistent with such a possibility, a recent study demonstrated the induction of SPRY2, but not SPRY1, downstream of BCR signaling in mouse B cells.15 This study also showed that SPRY2 levels negatively correlate with Erk signaling in mouse B cells, a finding similar to that described in other cellular systems.9,10,15 However, the molecular mechanism by which SPRY2 functions as a negative regulator of BCR signaling has not been deciphered. Moreover, the role of SPRY2 in B-cell development and function are unknown. SPRY2 was previously shown to be downregulated in diffuse large B-cell lymphoma but the functional significance of this downregulation remains ambiguous.15
In the present study, we identify SPRY2 downregulation as a marker of poor prognosis in CLL and demonstrate that the loss of SPRY2 provides a novel mechanism to constitutively activate BCR and MAPK-Erk signaling in CLL through spleen tyrosine kinase (Syk). Finally, we show that SPRY2 is targeted by microRNA-21 (miR-21) in poor-prognosis CLL that leads to a constitutively activated state of BCR and MAPK-Erk signaling in CLL cells.
Methods
Isolation of CLL cells from patients and normal B (nB) cells from healthy donors
Peripheral blood (PB) from CLL patients/healthy donors was obtained under an approved Institutional Review Board protocol. Mononuclear cells were isolated using Lymphoprep (Stemcell Technologies) following manufacturer’s instruction. CLL/nB cells were isolated by negative selection using CLL cells/B-cell Isolation Kit (Miltenyi Biotech). The purity of the isolated CLL/nB cells was tested by flow cytometry using CD19+CD5+/only CD19+ cell surface markers. When the CLL cell number was more than 90% in the PB from patients, the cell purification step was not performed.
Calcium mobilization assay
Calcium influx assay of human nB cells from PB and murine splenic B cells was performed and analyzed as described.16
Animal studies
CD19-cre animals were kindly provided by Dr Runqing Lu, University of Nebraska Medical Center (UNMC). Spry2 conditional transgenic mouse17 was purchased from the Mutant Mouse Regional Resource Center, University of North Carolina. To generate CD19-cre;Spry2(tg), CD19-cre and Spry2(tg)flox/flox mice were crossed. Over-expression of spry2 in B cells was tested by western blot. Expression of spry2 was tracked by green fluorescent protein (GFP)+ cells in flow cytometry experiments. Nonobese diabetic-severe combined immunodeficiency γ-chain–deficient (NSG) mice were purchased from The Jackson Laboratory. All animal experiments were conducted under the Institutional Animal Care and Use Committee-approved protocols.
Nucleofection
Western blot analysis
As previously described, western blotting was performed to analyze protein expression.20 (See supplemental Methods, available on the Blood Web site).
Results
Spry2 expression is lower in CLL cells from poor-prognosis patients
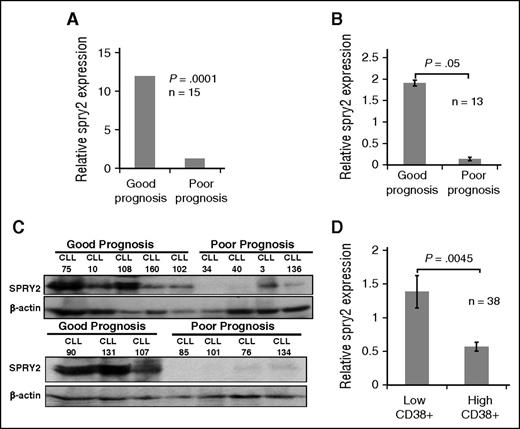
CLL is clinically heterogeneous, with varying clinical outcomes and possibly distinct molecular pathogenesis. In order to identify molecular mechanisms associated with poor-prognosis CLL, we performed RNA-sequencing–based transcriptome analysis of 15 CLL samples: 7 from good-prognosis and 8 from poor-prognosis patients. Poor-prognosis patients were defined by unmutated IgVH segments, Ch11q22 deletion, Ch17p deletion, trisomy Ch12, and/or high CD38 expression. Good-prognosis patients were defined as having mutated IgVH, Ch13q14 deletions, and/or low CD38 expression (criteria used for Figure 1A-C). Using these approaches, the expression levels of 146 genes were found to be significantly different between good- vs poor-prognosis CLL samples. We further selected genes based on their roles in B-cell biology, or their putative tumor suppressor or pro-oncogenic functions. Interestingly, spry2 was found to be significantly downregulated (3.4 log twofold) in the CLL cells from poor-prognosis patients compared with those from good-prognosis patients (Figure 1A). The differential expression was confirmed using western blotting and real-time polymerase chain reaction of CLL cells from good- vs poor-prognosis patients (Figure 1B-C). High CD38 positivity of CLL cells is associated with poor patient outcomes and constitutive activation of MAPK-Erk signaling.21 Therefore, we further compared spry2 expression in a larger cohort of 38 patients. Spry2 was significantly downregulated (∼2.8-fold) in CLL cells of patients with high CD38 expression compared with those with low CD38 expression (Figure 1D). Examination of the expression of other Spry family members (SPRY1, 3, and 4) in our data set showed that none of them were significantly differentially expressed between the good- and poor-prognosis CLL cells (data not shown). Thus, our results show that the expression of SPRY2 is significantly downregulated in CLL cells from patients with poorer outcomes.
Comparison of SPRY2 levels in CLL cells from good- and poor-prognosis patients. To compare the expression of SPRY2, CLL cells were isolated from PB of good- and poor-prognosis patients. (A) Relative messenger RNA level of spry2 from transcriptome analyses of 7 good-prognosis and 8 poor-prognosis CLL patients (n = 15). RNA isolated from PB CLL cells was used for sequencing. The expression was normalized with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and genomic reference DNA was used as control for transcriptome analysis. (B) Real-time polymerase chain reaction measurement of relative expression of spry2 in CLL cells from good- and poor-prognosis patients, normalized with GAPDH. (C) Levels of SPRY2 protein expression was measured in good-prognosis patients (n = 8) and poor-prognosis patients (n = 8). Displayed is a scanned western blot showing reduced protein levels of SPRY2 in poor-prognosis CLL patients. Mononuclear cells from healthy donors were used as positive control for antibody. A total of 50 μg of protein was loaded on 10% sodium dodecyl sulfate gel and β-actin was used as loading control. (D) Microarray data showing low relative expression of SPRY2 in patients with high CD38 expression. Patients with >30% of CD38-positive cells were considered CD38 high (n = 15) and patients with <30% were considered low CD38 (n = 23). GAPDH was used to normalize the value. P = .0045.
Comparison of SPRY2 levels in CLL cells from good- and poor-prognosis patients. To compare the expression of SPRY2, CLL cells were isolated from PB of good- and poor-prognosis patients. (A) Relative messenger RNA level of spry2 from transcriptome analyses of 7 good-prognosis and 8 poor-prognosis CLL patients (n = 15). RNA isolated from PB CLL cells was used for sequencing. The expression was normalized with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and genomic reference DNA was used as control for transcriptome analysis. (B) Real-time polymerase chain reaction measurement of relative expression of spry2 in CLL cells from good- and poor-prognosis patients, normalized with GAPDH. (C) Levels of SPRY2 protein expression was measured in good-prognosis patients (n = 8) and poor-prognosis patients (n = 8). Displayed is a scanned western blot showing reduced protein levels of SPRY2 in poor-prognosis CLL patients. Mononuclear cells from healthy donors were used as positive control for antibody. A total of 50 μg of protein was loaded on 10% sodium dodecyl sulfate gel and β-actin was used as loading control. (D) Microarray data showing low relative expression of SPRY2 in patients with high CD38 expression. Patients with >30% of CD38-positive cells were considered CD38 high (n = 15) and patients with <30% were considered low CD38 (n = 23). GAPDH was used to normalize the value. P = .0045.
SPRY2 is induced upon BCR crosslinking of nB cells and CLL cells, and functions as a negative-regulator of BCR signaling
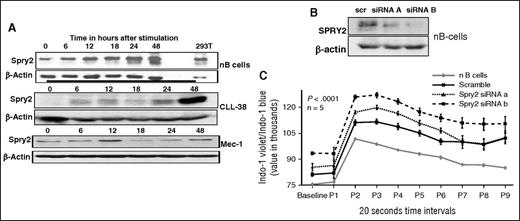
It is well established that BCR signaling is critical for survival and proliferation of CLL cells; however, no known recurrent mutations have so far been identified in this pathway among CLL patients. Moreover, it is known that SPRY2 functions as a negative-feedback regulator of RTKs.22-25 To investigate if SPRY2 is regulated by BCR signaling, we stimulated nB cells and CLL cells using BCR-crosslinking with 5 μg of anti-IgM/IgD antibody. Cells were collected at 0, 6, 12, 24, and 48 hours after stimulation and the expression of SPRY2 was determined by western blotting. We observed a gradual increase over time in SPRY2 expression in BCR-stimulated nB cells (Figure 2A, top). On the other hand, primary CLL cells and the Mec-1 CLL cell line exhibited a biphasic induction of SPRY2 (Figure 2A, middle and bottom). In CLL cells, SPRY2 expression peaked at 12 hours upon stimulation and declined at later time points. Thus, we conclude that SPRY2 expression induced by BCR-crosslinking is sustained in nB cells but follows a cyclical expression pattern in CLL cells and is not sustained.
Effect of SPRY2 on BCR signaling. To study the role of SPRY2 in CLL and nB cells, we isolated CLL and nB cells from patients and healthy donors, respectively. (A) nB cells, primary human CLL cells, and Mec-1 CLL cells were stimulated by BCR crosslinking for 0, 6, 12, 18, 24, and 48 hours. Cells were washed and protein lysate was prepared. Protein level of SPRY2 was determined by western blotting. SPRY2 levels in nB cells (top), SPRY2 levels in primary CLL cells from patient (middle), and SPRY2 levels in Mec-1 CLL cells (bottom) are shown. (B) To test the efficacy of siRNAs against SPRY2, nB cells were transfected with siRNAa and siRNAb after 48 hours of transfection cells, and were washed and lysate was prepared. An equal amount of protein was loaded in each well, and scramble siRNA and β-actin was used as control. Displayed is scanned western blot showing decrease in SPRY2 levels after siRNA treatment. (C) nB cells were isolated from healthy donors and nucleofected with scramble, siRNA A, and siRNA B. After 48 hours, calcium influx assay was performed using Indo-1 dye dots representing 20-second time intervals. Displayed is mean graph of Indo-1 violet/Indo-1 blue ratio, nB-cell samples from different healthy donors (n = 5), and P < .0001.
Effect of SPRY2 on BCR signaling. To study the role of SPRY2 in CLL and nB cells, we isolated CLL and nB cells from patients and healthy donors, respectively. (A) nB cells, primary human CLL cells, and Mec-1 CLL cells were stimulated by BCR crosslinking for 0, 6, 12, 18, 24, and 48 hours. Cells were washed and protein lysate was prepared. Protein level of SPRY2 was determined by western blotting. SPRY2 levels in nB cells (top), SPRY2 levels in primary CLL cells from patient (middle), and SPRY2 levels in Mec-1 CLL cells (bottom) are shown. (B) To test the efficacy of siRNAs against SPRY2, nB cells were transfected with siRNAa and siRNAb after 48 hours of transfection cells, and were washed and lysate was prepared. An equal amount of protein was loaded in each well, and scramble siRNA and β-actin was used as control. Displayed is scanned western blot showing decrease in SPRY2 levels after siRNA treatment. (C) nB cells were isolated from healthy donors and nucleofected with scramble, siRNA A, and siRNA B. After 48 hours, calcium influx assay was performed using Indo-1 dye dots representing 20-second time intervals. Displayed is mean graph of Indo-1 violet/Indo-1 blue ratio, nB-cell samples from different healthy donors (n = 5), and P < .0001.
We next studied the effect of downregulating SPRY2 expression on BCR signaling in nB cells. Compared with the scramble small interfering RNA (siRNA) control, both SPRY2 siRNAs led to a decrease in the SPRY2 protein levels (Figure 2B). Next, we transfected nB cells and compared their anti–IgM-induced calcium influx with that of unperturbed control following with the Indo-1 dye. Compared with untransfected and scrambled siRNA controls, B cells with SPRY2 knockdown exhibited elevated calcium influx (Figure 2C). Even the basal levels of calcium were increased in B cells upon SPRY2 knockdown. Taken together, these results establish that SPRY2 functions as a negative regulator of BCR signaling.
SPRY2 depletion in human CLL cells from good-prognosis patients lead to increased proliferation
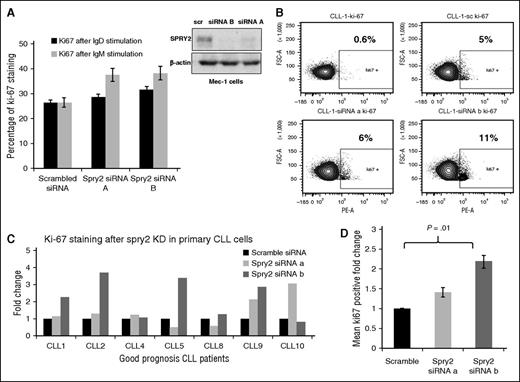
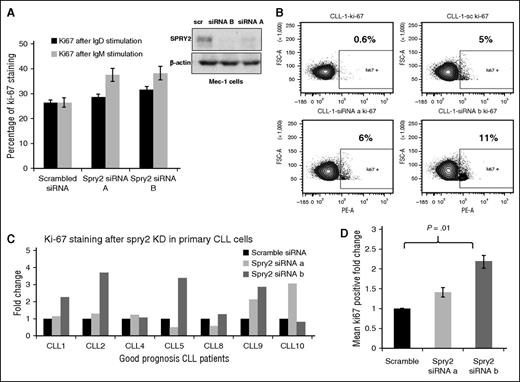
To assess if the downregulation of SPRY2 expression in poor- vs good-prognosis CLL cells is of functional consequence, we examined the impact of SPRY2 depletion in good-prognosis CLL cells. For this purpose, we isolated CLL cells from newly diagnosed patients with good-prognosis CLL. We also used the Mec-1 human CLL-cell line, a widely used cultured CLL-cell model, for these studies. Mec-1 cells have an intermediate level of SPRY2 relative to primary good- and poor-prognosis CLL cells (Figure 2A). Because SPRY2 expression is induced in CLL cells upon BCR stimulation (Figure 2A), we assessed the impact of SPRY2 depletion on anti-IgM/anti-IgD–induced Mec-1 cell proliferation, by analyzing changes in Ki-67+ fraction in fluorescence-activated cell sorter analyses. SPRY2 knockdown led to an increase in proliferation of Mec-1 cells relative to scrambled siRNA control under both anti-IgM and anti-IgD crosslinking conditions (Figure 3A). Next, we transfected primary human CLL cells isolated from PB of 7 different good-prognosis patients expressing higher levels of SPRY2 with siRNAs against SPRY2. At 24 hours posttransfection, the cells were subjected to BCR crosslinking and proliferation was assessed 48 hours later. Knockdown of SPRY2 led to an increase in the number of proliferating cells compared with the scrambled siRNA transfected cells (Figure 3B-D). We observed an increase in proliferation upon SPRY2 depletion compared with scrambled controls, with increased proliferation seen in 6 out of 7 with siRNA-B and in 5 out of 7 cases with siRNA-A–mediated knockdown. The effects on proliferation were consistent with a more robust SPRY2 knockdown with siRNA-B than siRNA-A (Figure 3A). SPRY2 knockdown in these cells also showed decrease in survival; however, the values did not reach statistical significance. This might be due to the use of anti-IgM antibodies for induction of SPRY2 expression as anti–IgM-stimulation itself has a positive effect on CLL-cell survival. Therefore, we reason that differences in the effects on survival may have been masked by anti-IgM stimulation. However, more CLL samples need to be analyzed to confirm these findings. These findings indicate that SPRY2 functions as a key negative feedback regulator of BCR signaling in CLL cells, thus limiting their BCR-induced proliferation.
Knockdown of SPRY2 increases proliferation of CLL cells from good-prognosis patients. To determine the effect of SPRY2 knockdown on human CLL cells, we used Mec-1 cells and primary CLL cells from good-prognosis CLL patients. (A) Mean bar graph of 3 repeats showing Ki-67 staining of Mec-1 cells after SPRY2 knockdown with anti-IgM and anti-IgD antibody stimulation (left) and scanned western blot (right), showing decrease in SPRY2 protein levels after siRNAs treatment. (B) Dot plot of patient’s sample CLL-1 showing an increase in proliferation after SPRY2 knockdown using two different siRNA. (C) CLL cells were isolated from PB of different good-prognosis CLL patients (n = 7). CLL cells were nucleofected with scramble, siRNA A and siRNA B, and coculture on S-17 stromal layer. After 48 hours, CLL cells were stained with Ki-67 stain and proliferation was measured. Displayed bar graph is fold in the rate of proliferation of CLL cells. (D) Mean fold change of (C) showing significant (P = .01) increase in proliferation after SPRY2 knockdown with siRNA B.
Knockdown of SPRY2 increases proliferation of CLL cells from good-prognosis patients. To determine the effect of SPRY2 knockdown on human CLL cells, we used Mec-1 cells and primary CLL cells from good-prognosis CLL patients. (A) Mean bar graph of 3 repeats showing Ki-67 staining of Mec-1 cells after SPRY2 knockdown with anti-IgM and anti-IgD antibody stimulation (left) and scanned western blot (right), showing decrease in SPRY2 protein levels after siRNAs treatment. (B) Dot plot of patient’s sample CLL-1 showing an increase in proliferation after SPRY2 knockdown using two different siRNA. (C) CLL cells were isolated from PB of different good-prognosis CLL patients (n = 7). CLL cells were nucleofected with scramble, siRNA A and siRNA B, and coculture on S-17 stromal layer. After 48 hours, CLL cells were stained with Ki-67 stain and proliferation was measured. Displayed bar graph is fold in the rate of proliferation of CLL cells. (D) Mean fold change of (C) showing significant (P = .01) increase in proliferation after SPRY2 knockdown with siRNA B.
SPRY2 expression induces apoptosis in human CLL cells from poor-prognosis patients
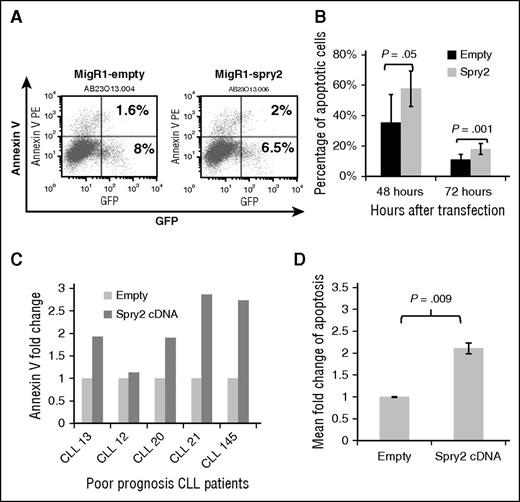
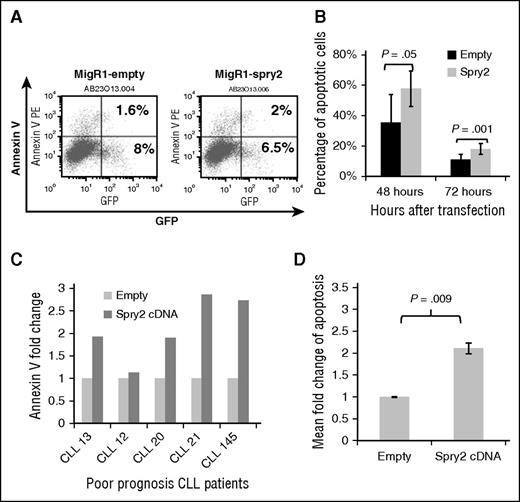
To further elucidate the underlying basis for the downregulation of SPRY2 expression in CLL cells from poor-prognosis patients, we also examined the impact of ectopic SPRY2 expression on CLL cells with low SPRY2 expression. First, we examined the impact of increasing the expression of SPRY2 by transfecting Mec-1 cells with a SPRY2 construct co-expressing GFP. At 48 and 72 hours posttransfection, we observed an average of 58% and 18% of SPRY2-GFP+–expressing Mec-1 cells to be Annexin V+, respectively, compared with 36% and 11% (Figure 4A-B). Next, we isolated CLL cells from 5 different poor-prognosis patients, ectopically expressed Spry2 to measure the proportion of Annexin V+ cells after 48 hours (Figure 4C). An increase in apoptosis was observed in CLL cells from all patients (Figure 4C-D). Moreover, we did not observe a significant impact on the proliferation of these cells compared with controls probably because these cells were not stimulated with anti-IgM antibodies. We choose not to stimulate these cells with anti-IgM because BCR stimulation through anti-IgM would have further induced SPRY2 levels. Thus, our results demonstrate that low levels of SPRY2 in poor-prognosis patients contribute to a survival advantage for CLL cells.
Expression of SPRY2 induces spontaneous apoptosis in CLL cells. (A) Mec-1 cells were transfected with empty and spry2-cDNA GFP co-expressing vectors. (B) Annexin V staining done after 48 and 72 hours. Only “live” cells were gated for the analysis. The numbers represent the percentage of GFP-positive cells also positive for Annexin V. (C) Primary CLL cells from different poor-prognosis CLL patients were nucleofected with spry2-cDNA and empty vector (n = 5). Bar graph represents fold in percentage of apoptotic cells. (D) Mean fold change of spry2-cDNA and empty vector transfected cells from (C). P = .009.
Expression of SPRY2 induces spontaneous apoptosis in CLL cells. (A) Mec-1 cells were transfected with empty and spry2-cDNA GFP co-expressing vectors. (B) Annexin V staining done after 48 and 72 hours. Only “live” cells were gated for the analysis. The numbers represent the percentage of GFP-positive cells also positive for Annexin V. (C) Primary CLL cells from different poor-prognosis CLL patients were nucleofected with spry2-cDNA and empty vector (n = 5). Bar graph represents fold in percentage of apoptotic cells. (D) Mean fold change of spry2-cDNA and empty vector transfected cells from (C). P = .009.
B-cell–specific elevation of SPRY2 levels attenuates and suppresses the B1-cell population
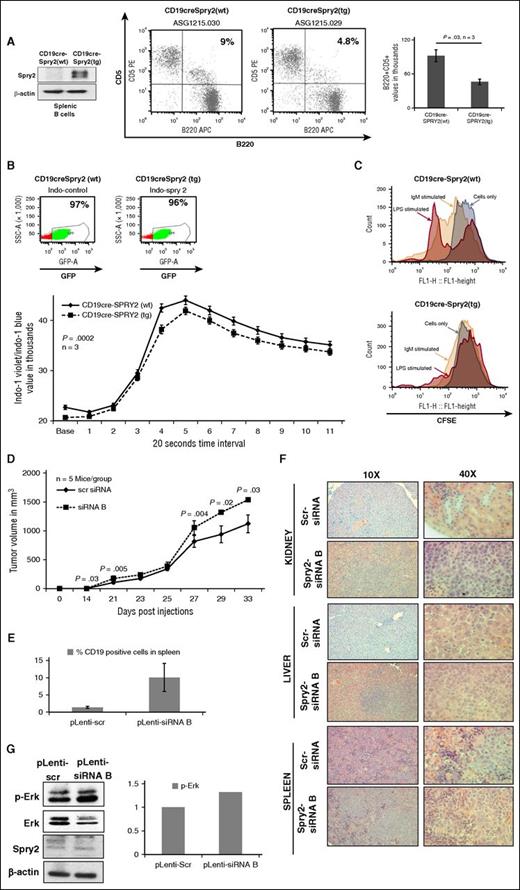
To study the effect of SPRY2 on the development and function of B cells in vivo, we generated a B-cell–specific SPRY2 transgenic mouse model. For this purpose, we crossed the mice harboring a spry2 transgene preceded by a STOP cassette (Spry2[tg]) with mice carrying a CD19-cre transgene to obtain a mouse line (CD19-cre;Spry2[tg]), in which the expression of cre-recombinase and SPRY2 are specifically induced in B cells (Figure 5A). We observed that the generation of B cells in these mice was normal (supplemental Figure 2A). However, we observed a decrease in the number of a specialized B-cell subset known as B1 cells in the CD19-cre;Spry2(tg) mice. This was particularly interesting because B1 cells are presumed to serve as precursors of CLL cells in mice. We found a decrease in the percentage of B1a cells (B220+CD5+) in the peritoneal cavity of these mice (Figure 5A). Furthermore, we examined the effect of SPRY2 overexpression on BCR signaling in B cells of CD19-cre;Spry2(tg) mice compared with CD19-cre control mice, by measuring their calcium influx over time in response to BCR crosslinking. Indeed, the B cells from CD19-cre;Spry2(tg) mice showed a slight but statistically significant reduction in calcium influx compared with B cells from CD19-cre control mice (Figure 5B). To study the effect of SPRY2 overexpression on proliferation, we isolated splenic B cells from CD19-cre;Spry2(tg) mice, labeled them with carboxyfluorescein diacetate succinimidyl ester (CFSE) dye, and cultured the cells with and without anti-IgM antibodies and lipopolysaccharide. Strikingly, we detected very few or no cells undergoing division in CD19-cre;Spry2(tg) compared with CD19-cre control cells with or without BCR stimulation (Figure 5C). Taken together, these results show that Spry2 functions to limit the numbers of B1 cells (CLL precursors) in mice and negatively regulates BCR signaling and associated proliferation in mouse B cells.
Effect of SPRY2 on B1-cell development, and BCR signaling in murine model and SPRY2 knockdown leads to disease progression in NSG mice. (A) Western blot showing overexpression of spry2 in splenic B cells of CD19-cre;Spry2(tg) mice (left). B1 cells were isolated from the peritoneal cavity of CD19-cre;Spry2(wt) and CD19-cre;Spry2(tg) mice. B1 cells were stained with CD5 and B220 dye to determine the frequency of B1 cells using flow cytometric analysis. Displayed is a dot plot showing B1a-cells frequency in CD19-cre;Spry2(wt) and CD19-cre;Spry2(tg) mice (middle). Bar graph of absolute number of B1a cells in the peritoneal cavity of CD19-cre;Spry2(wt) and CD19-cre;Spry2(tg) mice (right). (B) Splenic B cells were isolated by negative selection from rtTA-positive CD19-cre;Spry2(wt) (top, left) and CD19-cre;Spry2(tg) (top, right) mice. GFP+ population displays CD19-cre–expressed cells and displayed is calcium mobilization assay of splenic B cells from above described mice (bottom). n = 3; P = .0002. (C) Splenic B cells were isolated from CD19-cre;Spry2(wt) and CD19-cre;Spry2(tg) mice, stained with CFSE dye and cultured in vitro for 6 days with and without anti-IgM antibody and LPS. Displayed is the CFSE labeling of splenic B cells of CD19-cre;Spry2(wt) (top) and CD19-cre;Spry2(tg) (bottom) mice. SPRY2 was stably knocked down in Mec-1 CLL cells using pLenti-siRNA A and pLenti-siRNA B, and pLenti-scr was used as control. (D) Average of tumor volume in mice in each group were transplanted with 1.5 million pLenti-scr and pLenti-siRNA B Mec-1 CLL cells (n = 5). Tumor volume was measured using a digital caliper, and we determined the length and width of the tumor. Tumor volume was calculated by V = (L × W × W) / 2 where V is the tumor volume, L is the tumor length, and W is the tumor width. (E) Frequency of human CD19+ cells in spleen of pLenti-scr and pLenti-siRNA B CLL-cell–transplanted mice. (F) Hematoxylin and eosin staining with tissue section of kidney, liver, and spleen to observe the number of CLL-cell infiltration in pLenti-scr and pLenti-siRNA B CLL-cell–transplanted mice. (G) Protein lysates were prepared from tumors; equal amount of protein was loaded in each lane of 10% sodium dodecyl sulfate gel. Shown is scanned western blot to determine the protein levels of p-Erk and SPRY2 in tumors from pLenti-scr and pLenti-siRNA B CLL-cell–transplanted mice. Total Erk and β-actin were used as control. Densitometric measurements showing elevated p-Erk, normalized by total Erk in SPRY2 knockdown tumors. LPS, lipopolysaccharide; rtTA, reverse tetracycline transactivator; WT, wild-type.
Effect of SPRY2 on B1-cell development, and BCR signaling in murine model and SPRY2 knockdown leads to disease progression in NSG mice. (A) Western blot showing overexpression of spry2 in splenic B cells of CD19-cre;Spry2(tg) mice (left). B1 cells were isolated from the peritoneal cavity of CD19-cre;Spry2(wt) and CD19-cre;Spry2(tg) mice. B1 cells were stained with CD5 and B220 dye to determine the frequency of B1 cells using flow cytometric analysis. Displayed is a dot plot showing B1a-cells frequency in CD19-cre;Spry2(wt) and CD19-cre;Spry2(tg) mice (middle). Bar graph of absolute number of B1a cells in the peritoneal cavity of CD19-cre;Spry2(wt) and CD19-cre;Spry2(tg) mice (right). (B) Splenic B cells were isolated by negative selection from rtTA-positive CD19-cre;Spry2(wt) (top, left) and CD19-cre;Spry2(tg) (top, right) mice. GFP+ population displays CD19-cre–expressed cells and displayed is calcium mobilization assay of splenic B cells from above described mice (bottom). n = 3; P = .0002. (C) Splenic B cells were isolated from CD19-cre;Spry2(wt) and CD19-cre;Spry2(tg) mice, stained with CFSE dye and cultured in vitro for 6 days with and without anti-IgM antibody and LPS. Displayed is the CFSE labeling of splenic B cells of CD19-cre;Spry2(wt) (top) and CD19-cre;Spry2(tg) (bottom) mice. SPRY2 was stably knocked down in Mec-1 CLL cells using pLenti-siRNA A and pLenti-siRNA B, and pLenti-scr was used as control. (D) Average of tumor volume in mice in each group were transplanted with 1.5 million pLenti-scr and pLenti-siRNA B Mec-1 CLL cells (n = 5). Tumor volume was measured using a digital caliper, and we determined the length and width of the tumor. Tumor volume was calculated by V = (L × W × W) / 2 where V is the tumor volume, L is the tumor length, and W is the tumor width. (E) Frequency of human CD19+ cells in spleen of pLenti-scr and pLenti-siRNA B CLL-cell–transplanted mice. (F) Hematoxylin and eosin staining with tissue section of kidney, liver, and spleen to observe the number of CLL-cell infiltration in pLenti-scr and pLenti-siRNA B CLL-cell–transplanted mice. (G) Protein lysates were prepared from tumors; equal amount of protein was loaded in each lane of 10% sodium dodecyl sulfate gel. Shown is scanned western blot to determine the protein levels of p-Erk and SPRY2 in tumors from pLenti-scr and pLenti-siRNA B CLL-cell–transplanted mice. Total Erk and β-actin were used as control. Densitometric measurements showing elevated p-Erk, normalized by total Erk in SPRY2 knockdown tumors. LPS, lipopolysaccharide; rtTA, reverse tetracycline transactivator; WT, wild-type.
SPRY2 depletion in CLL cells results in more rapid lymphomagenesis in NSG mice
In view of the effect of SPRY2 on the survival and proliferation in vitro, as well as its impact in regulating the B1-cell pool in mice, we modeled the low SPRY2 expression seen in human CLL in a mouse lymphomagenesis model. For this purpose, we used a xenograft model with Mec-1 CLL cells transplanted into NSG mice. We derived SPRY2 knockdown version of Mec-1 cells by transducing them with pLenti-siRNA(A) or pLenti-siRNA(B), with the pLenti-scramble (pLenti-scr) siRNA Mec-1 cell line as a control. We observed a significant increase in proliferation and decrease in the percentage of cells exhibiting apoptosis in pLenti-siRNA(A) and pLenti-siRNA(B) cell lines, compared with scrambled control (supplemental Figure 2B-C). Next, we injected 1.5 × 106 pLenti-siRNA(B) (higher degree of SPRY2 downregulation) or pLenti-scr cells subcutaneously into the left flank of sublethally irradiated NSG mice (5 mice per group). Tumors were palpable 21 days post-injection. Notably, pLenti-siRNA(B) cells gave rise to significantly larger tumors at the primary site of injection (Figure 5D). Dissemination of human CLL cells was significantly increased in the spleens of pLenti-siRNA(B) cell-injected mice as measured by anti-human CD19 staining (Figure 5E). Hematoxylin and eosin staining of the spleen, liver, and kidney also revealed more organ infiltration by pLenti-siRNA(B)-expressing CLL cells (Figure 5F). Western blot analysis of tumors isolated from NSG recipient mice confirmed downregulation of SPRY2 in pLenti-siRNA(B) cell-derived tumors compared with control cell-derived tumors (Figure 5G). Furthermore, we also observed an increase in phosphorylated-ERK (p-ERK) levels in tumors arising from SPRY2-depleted Mec-1 cells (Figure 5G). These results show that lower SPRY2 expression leads to the formation of more rapid and aggressive lymphomagenesis in mice, indicating a role for SPRY2 downregulation in CLL disease progression.
SPRY2 interacts with RAF-1, BRAF, and Syk to downregulate MAPK-Erk signaling in CLL cells
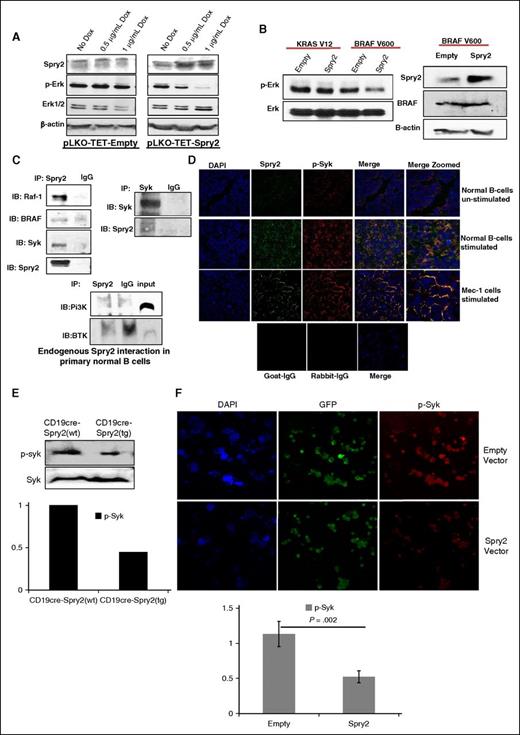
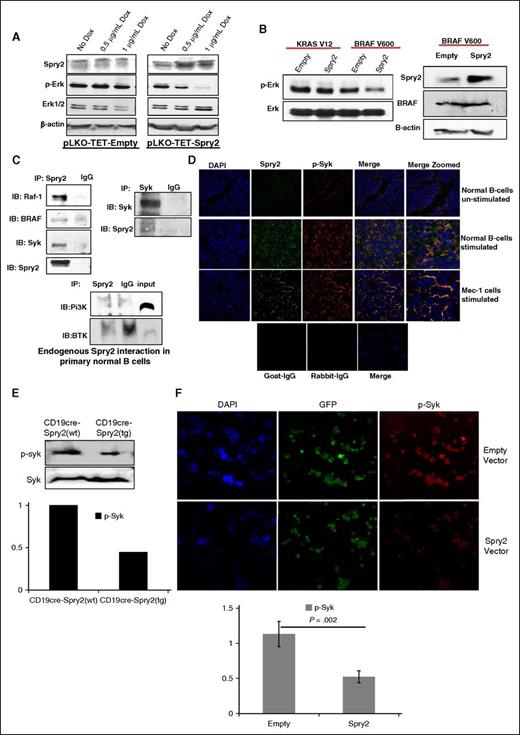
To elucidate the mechanism by which SPRY2 mediates a decrease in proliferation and survival of human CLL cells, we used a Tet-On Inducible System in Mec-1 cells. Upon doxycycline (DOX) treatment, we observed a dose-dependent increase in SPRY2 expression, which was accompanied by a decrease in p-ERK levels (Figure 6A). Other groups have shown that SPRY2 interacts with RAF-1/BRAF to inhibit MAPK-Erk signaling in malignancies such as multiple myeloma.26,27 Interestingly, mutations in RAF-1 and BRAF have also been identified in CLL patients.6 We first tested the conservation of these networks in humans using immunoprecipitation of SPRY2 in BCR-stimulated nB cells from healthy donors, which showed co-immunoprecipitation of RAF-1 and BRAF, suggesting that SPRY2 may attenuate MAPK-Erk signaling by inhibiting RAF-1 and BRAF activities.
SPRY2 downregulate MAPK-ERK signaling by interacting with RAF-1, Syk, and BRAF. (A) Mec-1 cells were transfected with pLKO-TET-empty and pLKO-TET-Spry2. Cells were treated with DOX for 4 days to induce the expression of SPRY2. Displayed is a scanned western blot of CLL cells transfected with empty and spry2 cDNA to measure the protein levels of SPRY2 and p-Erk upon DOX treatment. β-actin and PAN (total) Erk1/2 were used as controls. (B) KRAS-V12 and BRAF-V600 mutants were co-transfected with empty and Spry2 vectors in Mec-1 cells. Scanned western blot showing the p-Erk and Erk levels in these cells (left). Scanned western blot of SPRY2 and BRAF protein levels in the same cells (right); β-actin was used as loading control. (C) Human nB cells were isolated from healthy donors and cells were stimulated by BCR crosslinking for 24 hours. Cells were lysed to prepare lysate for immunoprecipitation (IP) (described in supplemental Methods). Displayed is a scanned western blot of immunoprecipitation with SPRY2 and Syk showing pull-down of RAF-1, Syk, BRAF, and SPRY2. IgG, Pi3K, and BTK were used as negative controls. (D) nB cells and Mec-1 cells were stimulated as described in (C) and cyotospin were prepared using these cells. Cells were stained with SPRY2, p-Syk, and DAPI fluorescence antibody. Displayed are immunofluorescence images showing co-localization of SPRY2 and p-Syk in nB cells and Mec-1 CLL cells. Unstimulated cells, goat-IgG, and rabbit-IgG were used as control. (E) nB cells were isolated from spleen of CD19-cre;Spry2(wt) and CD19-cre;Spry2(tg) mice, and stimulated for 30 minutes with anti-IgM antibody. Displayed is scanned western blot of p-Syk and Syk, and densitometric measurements showing normalized decreased levels of p-Syk. (F) Mec-1 CLL cells were overexpressed with Spry2 cDNA and empty vector co-expressing GFP; cytospins were prepared of these cells. Slides were stained with p-Syk (red) antibody. Displayed are immunofluorescence images and densitometric measurements showing decreased levels of normalized p-Syk in SPRY2 co-expressing GFP-positive cells. DAPI, 4,6 diamidino-2-phenylindole; IB, immunoblotting.
SPRY2 downregulate MAPK-ERK signaling by interacting with RAF-1, Syk, and BRAF. (A) Mec-1 cells were transfected with pLKO-TET-empty and pLKO-TET-Spry2. Cells were treated with DOX for 4 days to induce the expression of SPRY2. Displayed is a scanned western blot of CLL cells transfected with empty and spry2 cDNA to measure the protein levels of SPRY2 and p-Erk upon DOX treatment. β-actin and PAN (total) Erk1/2 were used as controls. (B) KRAS-V12 and BRAF-V600 mutants were co-transfected with empty and Spry2 vectors in Mec-1 cells. Scanned western blot showing the p-Erk and Erk levels in these cells (left). Scanned western blot of SPRY2 and BRAF protein levels in the same cells (right); β-actin was used as loading control. (C) Human nB cells were isolated from healthy donors and cells were stimulated by BCR crosslinking for 24 hours. Cells were lysed to prepare lysate for immunoprecipitation (IP) (described in supplemental Methods). Displayed is a scanned western blot of immunoprecipitation with SPRY2 and Syk showing pull-down of RAF-1, Syk, BRAF, and SPRY2. IgG, Pi3K, and BTK were used as negative controls. (D) nB cells and Mec-1 cells were stimulated as described in (C) and cyotospin were prepared using these cells. Cells were stained with SPRY2, p-Syk, and DAPI fluorescence antibody. Displayed are immunofluorescence images showing co-localization of SPRY2 and p-Syk in nB cells and Mec-1 CLL cells. Unstimulated cells, goat-IgG, and rabbit-IgG were used as control. (E) nB cells were isolated from spleen of CD19-cre;Spry2(wt) and CD19-cre;Spry2(tg) mice, and stimulated for 30 minutes with anti-IgM antibody. Displayed is scanned western blot of p-Syk and Syk, and densitometric measurements showing normalized decreased levels of p-Syk. (F) Mec-1 CLL cells were overexpressed with Spry2 cDNA and empty vector co-expressing GFP; cytospins were prepared of these cells. Slides were stained with p-Syk (red) antibody. Displayed are immunofluorescence images and densitometric measurements showing decreased levels of normalized p-Syk in SPRY2 co-expressing GFP-positive cells. DAPI, 4,6 diamidino-2-phenylindole; IB, immunoblotting.
It has been demonstrated that BRAF-V600E mutant harboring a valine to glutamic acid substitution does not physically interact with SPRY2 and is resistant to SPRY2-mediated attenuation of MAPK-Erk signaling.28 Additionally, RAS mutants function upstream to RAF and can bypass SPRY2-mediated inhibition.29 Therefore, we tested the effect of SPRY2 on these mutants in human CLL cells. Intriguingly, SPRY2 expression led to a decrease in the p-Erk expression level even in the presence of the BRAF-V600 mutant; although however, there was no impact of inducing SPRY2 expression in KRAS-V12 mutant transfected cells (Figure 6B). These results indicate an additional and possibly parallel mechanism by which SPRY2 may attenuate MAPK-Erk signaling in both B cells and CLL cells.
Interestingly, a recent study showed that in the presence of BRAF inhibitors, Syk undergoes upregulation to activate Erk signaling in CLL cells, an event mediated via RAS.30 Furthermore, inhibition of Syk reversed the Erk hyperactivation and led to a decrease in proliferation of CLL cells.30 Therefore, we speculated that SPRY2, in the presence of RAF inhibition may also inhibit Syk activity in B cells and CLL cells to attenuate MAPK-Erk signaling. To test this hypothesis, we performed immunoprecipitation assays as described in supplemental Methods. Intriguingly, SPRY2 was found to physically interact with Syk but not with Bruton tyrosine kinase (BTK) and PI3K in B cells as determined by reciprocal immunoprecipitation experiments (Figure 6C). Also, using immunofluorescence analysis, we observed a co-localization of SPRY2 with p-Syk in nB cells and Mec-1 CLL cells (Figure 6D). We next tested the effect of SPRY2 overexpression on the activated form of Syk by measuring the levels of p-Syk in splenic B cells from CD19-cre;Spry2(tg) mice and SPRY2 overexpressing Mec-1 cells by immunofluorescence. Notably, we observed a decrease in p-Syk expression in B cells of CD19-cre;Spry2(tg) mice and Mec-1 cells overexpressing SPRY2 compared with B cells from CD19-cre mice and empty vector Mec-1 cells, respectively (Figure 6E-F). We also observed a synergistic effect of SPRY2 with BRAF and Syk inhibitors in the presence of the BRAF-V600 mutant, again highlighting the dual mechanism through with SPRY2 downregulates MAPK-Erk signaling in CLL cells (supplemental Figure 3). Thus, our results demonstrate that SPRY2 attenuates BCR-mediated MAPK-Erk signaling by simultaneous inhibition of RAF and Syk activity in both B cells and CLL cells.
Mir-21 targets SPRY2 in CLL cells to activate Syk and MAPK-Erk signaling
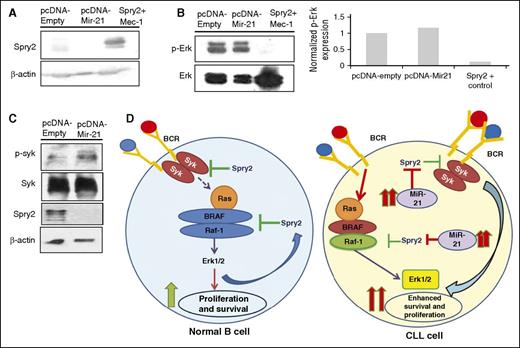
Given the SPRY2 downregulation in patients with poor-prognosis CLL, and a cyclic pattern of SPRY2 expression in BCR-crosslinked CLL cells (Figure 2), we reasoned that an epigenetic mechanism may deregulate SPRY2 expression in CLL. Although, the spry2 gene is hypermethylated in diffuse large B-cell lymphoma, Chen et al did not observe any hypermethylation of spry2 locus in patients with CLL.31 Therefore, we sought to identify alternate mechanisms for spry2 downregulation in poor-prognosis CLL patients. Interestingly, SPRY2 has been shown to be a direct target of miR-21 in other cellular systems.32-34 Furthermore, mir-21 is an oncomir that is highly overexpressed in CLL patients with poorer outcomes.35,36 Therefore, we overexpressed miR-21 in Mec-1 cells to study whether it can regulate Spry2 in CLL cells. Notably, we observed a decrease in SPRY2 expression in Mec-1 cells expressing high levels of miR-21 compared with those with an empty-vector control (Figure 7A). Intriguingly, concurrent with SPRY2 downregulation in miR-21 overexpressing Mec-1 cells, we also observed an increase in the levels of p-Syk and p-Erk (Figure 7B-D). In summary, our results demonstrate that Spry2 is deregulated by miR-21 in CLL cells. The results here also provide a mechanism by which miR-21 promotes CLL progression via downregulation of SPRY2.
miR-21 targets SPRY2 in human CLL cells to activate BCR-mediated MAPK-Erk signaling. (A) Scanned western blot showing SPRY2 protein levels in miR-21–overexpressing CLL cells; β-actin was used as loading control. (B) Protein levels of p-Erk and Erk in miR-21–overexpressing cells, and densitometric measurements showing elevated levels of p-Erk in miR-21–overexpressing CLL cells. (C) Scanned western blot showing the protein levels of p-Syk, Syk, and SPRY2 in miR-21–overexpressing Mec-1 CLL cells. (D) In the case of nB cells (left), BCR stimulation leads to activation of MAPK-Erk signaling, which results in their proliferation and survival. As a homeostasis response, SPRY2 gets induced to regulate signaling in activated B cells. Whereas in the case of CLL cells (right), elevated levels of miR-21 leads to decreased SPRY2, which results in constitutive activation of BCR and MAPK-Erk signaling. This in turn increases the proliferation and survival of CLL cells.
miR-21 targets SPRY2 in human CLL cells to activate BCR-mediated MAPK-Erk signaling. (A) Scanned western blot showing SPRY2 protein levels in miR-21–overexpressing CLL cells; β-actin was used as loading control. (B) Protein levels of p-Erk and Erk in miR-21–overexpressing cells, and densitometric measurements showing elevated levels of p-Erk in miR-21–overexpressing CLL cells. (C) Scanned western blot showing the protein levels of p-Syk, Syk, and SPRY2 in miR-21–overexpressing Mec-1 CLL cells. (D) In the case of nB cells (left), BCR stimulation leads to activation of MAPK-Erk signaling, which results in their proliferation and survival. As a homeostasis response, SPRY2 gets induced to regulate signaling in activated B cells. Whereas in the case of CLL cells (right), elevated levels of miR-21 leads to decreased SPRY2, which results in constitutive activation of BCR and MAPK-Erk signaling. This in turn increases the proliferation and survival of CLL cells.
Discussion
Clinical heterogeneity is a major problem in the management of CLL. The heterogeneous clinical outcome in patients appears to be the results of interactions between several molecules and cellular pathways. Consequently, in order to treat CLL effectively, a better understanding of the molecules and cellular pathways that contribute to such a heterogeneous clinical outcome is needed. In the present study, we have elucidated the molecular basis for aberrant BCR and MAPK-Erk signaling, where SPRY2 acts as a negative regulator for survival and proliferation of CLL cells. Moreover, SPRY2 may represent a molecule responsible for maintaining the clinical heterogeneity in CLL.
Notably, our findings identify SPRY2 as a negative-feedback regulator downstream to BCR stimulation that is critical for attenuation of MAPK-Erk signaling. Moreover, SPRY2 may function as an attenuator of tonic BCR signaling in CLL cells and B cells because basal levels of signaling are elevated upon SPRY2 knockdown. Although SPRY2 overexpression in CD19-cre;Spry2(tg) mice led to impaired BCR signaling in B cells, we did not observe an apparent defect in the overall generation of B cells. Notably though, and directly relevant to the role of SPRY2 downregulation in CLL, we observed a decrease in B1 cells. These results indicate a potential role for SPRY2 in the development of B1 cells and hence, possibly in the initiation of CLL.37 It will be of considerable interest to breed the CD19-cre;Spry2(tg) mice to established models of CLL, such as Eμ-Tcl1 or IRF4−/−Vh11 mice, to directly study the role of SPRY2 in the development of CLL.37 Interestingly, SPRY2 levels are downregulated in CLL cells isolated from the IRF4−/−Vh11 mouse model (data not shown). Even though no other defects in B-cell development are apparent in CD19-cre;Spry2(tg) mice, it will be interesting to further study the induction of functional humoral responses in these mice, given our findings that aberration of SPRY2 expression deregulate BCR signaling.
Functionally, our studies have shown that SPRY2-mediated regulation of BCR signaling is important for the survival and proliferation of CLL cells. Moreover, SPRY2 plays a role in controlling the disease aggressiveness as knockdown of SPRY2 in Mec-1 CLL cells resulted in more aggressive disease in NSG mice. We show that SPRY2 expression in nB cells and CLL cells leads to decreased p-Syk levels. Mechanistically, BCR signaling in CLL cells constitutes a signaling axis whereby Syk can also function to regulate the activation of MAPK-Erk signaling (Figure 7D). Of significant interest in this regard, we observed that SPRY2 not only interacts with and antagonizes RAF/BRAF activities but that it also interacts and co-localizes with Syk near the plasma membrane to disrupt the MAPK-Erk signaling axis. Therefore, we propose a model in which SPRY2 functions to regulate two different nodes of an overlapping signaling axis by attenuating Syk, as well as RAF/BRAF activity (Figure 7D). Importantly, our studies indicate that SPRY2 functions as a broad attenuator of BCR signaling as evidenced by a decrease in calcium influx upon BCR stimulation, a process mediated by PLCγ2 signaling downstream of Syk activation.
SPRY2 has been shown to interact with RTKs and the associated adaptor molecules through its SH2-domain–binding motifs generated upon its phosphorylation.10 Syk is a non-RTK harboring multiple SH2 domain. Hence, it is tempting to speculate that SPRY2 might interact with Syk using one or more of its SH2-domain–binding motifs. However, the precise domain(s) required for the interaction of SPRY2 with Syk is currently unknown. Additionally, how the functional inhibition of Syk is brought about by its interaction with Spry2 is an open question. Furthermore, SPRY2 overexpression in CLL cells induces an anti-survival effect that functions synergistically with Syk and BRAF inhibitors. Collectively, these studies highlight the presence of dual mechanisms through which SPRY2 regulates BCR-induced MAPK-Erk signaling in CLL and B cells (Figure 7D). Thus, our findings provide a strong rationale for targeting of these pathways in the treatment of CLL patients, in particular those with MAPK-pathway–associated mutations. A recently study has identified a small subset of CLL patients who do not respond to the Btk inhibitor ibrutinib.38 It will be of interest to evaluate the therapeutic potential of a combinatorial Syk and MAPK-Erk inhibition in such patients.
Spry2 is either epigenetically silenced or repressed by miR-21 in several cancers, including breast, prostrate, lungs, liver, and lymphoma.15,34,39-43 Chen et al have demonstrated that the promoter region of spry2 was only hypermethylated in a small fraction of the 55 CLL patients that were profiled, signifying alternate mechanisms that lead to Spry2 downregulation in CLL.31 Interestingly, we identify SPRY2 as a direct target of miR-21 in human CLL cells. Several studies have correlated high miR-21 expression in CLL patients with poorer outcomes.35,36 High miR-21 expression has been shown to activate MAPK-Erk signaling in several malignancies by suppressing SPRY2 levels.33,41 However, in this report, we have shown the molecular mechanism through which miR-21 leads to disease advancement. We observed elevated p-Syk and p-Erk levels and low levels of SPRY2 in high miR-21–expressing CLL cells. Together, these findings suggest that miR-21 targets SPRY2 to activate Syk-mediated BCR and MAPK-Erk signaling in poor-prognosis CLL. Also, miR-21 overexpression may be responsible for the biphasic expression of SPRY2 observed in CLL cells. Further studies are required to establish the robustness of the biphasic cyclical expression pattern of SPRY2 at later time points in CLL cells. To elucidate this further, the kinetics of miR-21 induction should be carefully monitored along with SPRY2 expression in CLL cells. Nevertheless, the biphasic expression of SPRY2 may contribute to the sustained BCR signaling in CLL cells leading to their enhanced survival and proliferation. Moreover, the molecular mechanisms leading to the upregulation of miR-21 in poor-prognosis CLL is still unknown.
Our studies show that SPRY2 functions as a molecular rheostat important for fine-tuning the signaling cascades critical for survival and proliferation of CLL. By investigating the relevance and mechanism of SPRY2 downregulation in human CLL cells and mouse models, our studies here identify and validate key molecular networks that can be therapeutically targeted in the treatment of CLL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Gail R. Martin for donating the Spry2(tg) mouse strain to the Mutant Mouse Regional Resource Center, and the UNMC Flow Core facility.
This work was supported by grants from the Department of Genetics, Cell Biology and Anatomy (UNMC); pilot grant funds from Dr Vimla Band; funding from Dr Robert T. Binhammer; grants from the National Institutes of Health, National Cancer Institute (CA87986 and CA105489) (H.B.); and graduate research assistance by UNMC (A.S.).
Authorship
Contribution: A.S. designed, performed experiments, analyzed data, and wrote the paper with the help of H.B., R.L., and S.S.J.; K.R., V.S., and N.K.C. performed experiments; R.G.B. provided CLL patients samples; S.J.P. provided and analyzed CLL samples; H.B. provided SPRY2 constructs and supervised experiments; R.L. provided Mec-1, the CD19-cre mouse model, and supervised experiments; and S.S.J. designed, analyzed data, and supervised experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shantaram S. Joshi, Department of Genetics, Cell Biology and Anatomy, University of Nebraska Medical Center, 986395 Nebraska Medical Center, Omaha, NE 68198-6395; e-mail: ssjoshi@unmc.edu.