In this issue of Blood, Hyvärinen et al1 show that mutant forms of complement factor H, which are commonly associated with atypical hemolytic uremic syndrome (aHUS), have impairments in binding to sialic acid on C3b-coated erythrocytes, platelets, and endothelial cells. The findings have implications in our understanding of the mechanisms underlying aHUS and the design of therapies for complement-mediated syndromes, infections, and cancer.
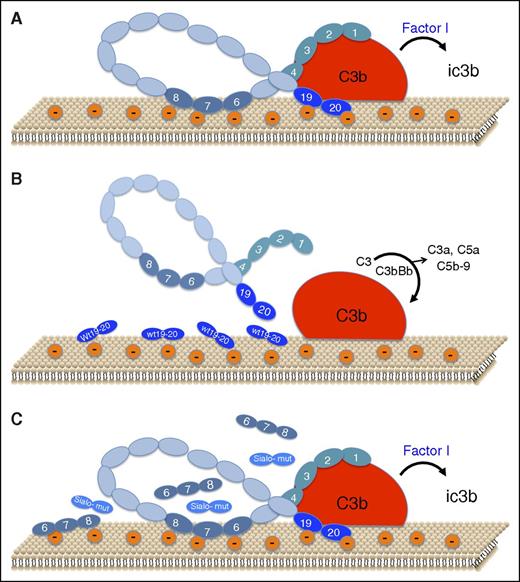
Schematic representation of the role of sialic acid recognition sites on factor H binding and function on the endothelial cell surface. (A) Under normal conditions, small amounts of C3b bind to host cells. CCP1-4 of factor H binds to C3b, and CCP19-20 binds to C3b and to polyanions (mostly sialic acid) on the cell surface, thereby forming a high-affinity ternary complex that facilitates factor I–mediated inactivation of C3b to iC3b and accelerated decay of any C3bBb that may form (not shown). CCP6-8 also binds to the cell surface via heparan sulfate. (B) Addition of exogenous wild-type CCP19-20 (wt 19-20) interferes with binding of factor H to the cell surface and to C3b, thereby allowing amplification of complement to proceed, with generation of the C3 convertase (C3bBb), liberation of anaphylatoxins C3a and C5a, and assembly of the lytic membrane attack complex, C5b-9. (C) Recombinant forms of CCP19-20 with aHUS-associated mutations at sialic acid recognition sites (Sialo-mut), fail to interfere with factor H binding to the polyanionic cell surface and to C3b, thereby allowing factor H to exhibit protective effects, as in panel A. CCP19-20 forms with mutations outside of the sialic acid recognition site behaved as in panel B. Addition of excess recombinant CCP6-8, known to bind to anionic heparan sulfate on the cell surface, also does not interfere with factor H binding or function, indicating the greater importance of sialic acid recognition by factor H.
Schematic representation of the role of sialic acid recognition sites on factor H binding and function on the endothelial cell surface. (A) Under normal conditions, small amounts of C3b bind to host cells. CCP1-4 of factor H binds to C3b, and CCP19-20 binds to C3b and to polyanions (mostly sialic acid) on the cell surface, thereby forming a high-affinity ternary complex that facilitates factor I–mediated inactivation of C3b to iC3b and accelerated decay of any C3bBb that may form (not shown). CCP6-8 also binds to the cell surface via heparan sulfate. (B) Addition of exogenous wild-type CCP19-20 (wt 19-20) interferes with binding of factor H to the cell surface and to C3b, thereby allowing amplification of complement to proceed, with generation of the C3 convertase (C3bBb), liberation of anaphylatoxins C3a and C5a, and assembly of the lytic membrane attack complex, C5b-9. (C) Recombinant forms of CCP19-20 with aHUS-associated mutations at sialic acid recognition sites (Sialo-mut), fail to interfere with factor H binding to the polyanionic cell surface and to C3b, thereby allowing factor H to exhibit protective effects, as in panel A. CCP19-20 forms with mutations outside of the sialic acid recognition site behaved as in panel B. Addition of excess recombinant CCP6-8, known to bind to anionic heparan sulfate on the cell surface, also does not interfere with factor H binding or function, indicating the greater importance of sialic acid recognition by factor H.
The complement system is a component of innate immunity, designed to eliminate invading pathogens while sparing healthy host cells. The alternative pathway of complement is constitutively active, continuously generating small amounts of C3b, an opsonin and key component of the C3bBb C3 convertase that is necessary for amplification and progression of the complement cascade. C3b binds indiscriminately to surfaces, whether they belong to pathogens or host cells. Thus, regulatory mechanisms that distinguish self vs nonself are required to prevent complement activation on healthy cells and unwanted tissue damage.
Factor H is an important negative regulator of complement that binds to C3b and host cell surfaces, where it competes with formation of the C3bBb convertase, accelerates its decay, and provides cofactor activity for C3b inactivation. Factor H mutations are the most common genetic cause of aHUS,2 a thrombotic microangiopathy with microvascular endothelial damage, hemolysis, thrombocytopenia, and renal dysfunction. Mutations of factor H are also linked to age-related macular degeneration and C3 glomerulopathies. Furthermore, host-derived factor H can be recruited by some pathogens and tumor cells to evade immune destruction. Thus, understanding how factor H interacts with C3b and the cell surface, and distinguishes self from nonself, has clinical relevance.
Factor H is an abundant plasma glycoprotein that comprises a string of 20 short consensus domains, complement control protein (CCP) repeats,3 that interact with C3b and cell surfaces. The current model holds that CCP1-4 binds to C3b and is necessary for cofactor and decay acceleration of C3bBb. CCP19-20 also binds to C3b but possesses recognition sites for polyanion-mediated binding to the cell surface. The majority of aHUS-associated factor H mutations are clustered in CCP19-20. Polyanions, such as glycosaminoglycans, heparan sulfate, and sialic acids, are expressed by all host cells, but rarely by pathogens, thereby distinguishing self from nonself.
Heparan sulfate is generally viewed as the main polyanion mediating factor H binding to endothelial cells.4 The problem is that the affinity of heparin to aHUS-associated factor H mutants correlates poorly with endothelial cell surface binding and anticomplement function. With this conundrum in mind, along with structural evidence that CCP19-20 contains conserved sialic acid binding sites linked to aHUS,5 Hyvärinen et al set out to delineate the role of this sugar moiety in factor H interactions with cells that participate in aHUS.
The investigators used recombinant CCP19-20 (referred to as FH19-201 ) in competition assays to assess effects on factor H binding and function (see figure). They first showed that wild-type CCP19-20 displaces factor H from C3b-coated erythrocytes, reversing the protection conferred by factor H against complement-mediated lysis. Conversely, excess CCP19-20 mutants L1189R and E1198A, which enhance heparin binding but impair interactions with sialic acid,5 had no effect on factor H binding or its ability to protect against lysis. Sialidase treatment of erythrocytes confirmed that a ternary complex of factor H, C3b, and sialic acid is essential for host cell protection, and that its assembly is impaired with CCP19-20 aHUS-associated mutants.
Because endothelial damage is a hallmark of aHUS, Hyvärinen et al also examined factor H binding and function on endothelial cells. The critical role of sialic acid was confirmed with sialidase and by blocking sialic acids with a sialic acid–binding lectin. As with erythrocytes, wild-type CCP19-20 disrupted factor H binding to C3b-coated cells and reduced factor H–mediated protection, an effect that was not observed with excess CCP19-20 mutants where the sialic acid binding site was disrupted but was seen with mutants that retain sialic acid binding. CCP6-8, which displays a heparan sulfate binding site, also did not displace factor H. Thus, the sialic acid/CCP19-20 interaction predominates in forming a stable complex with C3b. Experiments with platelets allowed similar conclusions to be drawn as with endothelial cells and erythrocytes.
What are the implications of these findings? For aHUS, the authors provide a unifying mechanism by which defects in factor H recognition of sialic acid on cell surfaces explain the involvement of erythrocytes, platelets, and endothelial cells. Thus, the anemia of aHUS, often ascribed to fragmentation, and the thrombocytopenia, usually attributed to consumption in platelet-fibrin aggregates, might be induced partly by complement-mediated destruction because of loss of factor H binding to sialic acid.
The potential relevance goes further. Sialic acids are diverse, varying in structure and expression level in different cells and tissues and in response to stresses and genetic factors.6 Several viruses and bacteria secrete sialidases that cleave sialic acids from host cell surfaces.7 By so limiting the protective properties of factor H, these sialic acid–modifying pathogens may “tip the balance,” triggering onset of symptomatic disease in aHUS caused by any mutation (not just factor H), as well as in diarrhea-associated HUS caused by enteropathogenic Escherichia coli or in HUS caused by the neuraminidase-secreting Streptococcus pneumoniae. That some patients with these latter forms of HUS respond to eculizumab supports the notion that loss of specific factor H–binding sialic acids might contribute to the tissue damage.8 Indeed, in the context of factor H, stress-induced changes in sialic acid amount and composition may impact on any disorder where excess complement activation participates.
The preceding raises several intriguing therapeutic considerations. Although modified forms of factor H, such as “mini-factor H” that comprise CCP1-4 and CCP19-20,9 are already being evaluated, could efficacy be enhanced by augmenting sialic acid binding? Might the recurrence frequency or severity of HUS be reduced with sialidase inhibitors?10 Conversely, can one safely and effectively interfere with factor H/sialic acid interactions with sialidases to prevent tumors and pathogens from acquiring factor H and thus evading host immunity?
Although there are numerous challenges in applying this new knowledge in glycomics to solve serious clinical problems, the mechanistic insights provided by Hyvärinen et al are a major step forward.
Conflict-of-interest disclosure: The author declares no competing financial interests.