In this issue of Blood, Claushuis et al report on a rigorous evaluation of the associations among baseline platelet counts, biomarkers of host response, and clinical outcomes in a large cohort of critically ill patients with sepsis.1
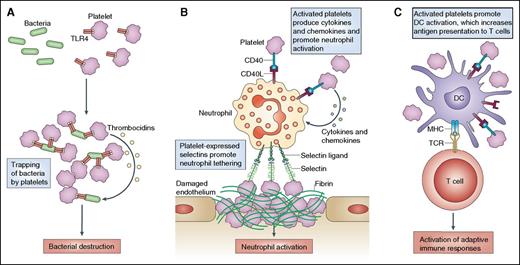
Three typical examples of how activated platelets can mediate cell–cell interactions and affect innate immune responses. (A) Platelet Toll-like receptor (TLR) expression enables activated platelets to bind and capture bacteria. Subsequently, the platelets may directly kill the bacteria by producing thrombocidins or by aggregating around the bacteria and “trapping” them for elimination by professional phagocytes. (B) It is now clear that platelets can also heterotypically interact with a wide variety of cells, including leukocytes. Activated platelets promote neutrophil tethering and activation through the expression of selectins, CD154 (also known as CD40L), and inflammatory cytokines and chemokines. (C) Similarly, activated platelets can promote the activation of monocytes and dendritic cells (DCs), particularly through CD40–CD154 interactions. This leads to increased antigen presentation to T cells and enhances adaptive immune responses. MHC, major histocompatibility complex; TCR, T-cell receptor. Reprinted from Semple et al,2 with permission from Macmillan Publishers Ltd.
Three typical examples of how activated platelets can mediate cell–cell interactions and affect innate immune responses. (A) Platelet Toll-like receptor (TLR) expression enables activated platelets to bind and capture bacteria. Subsequently, the platelets may directly kill the bacteria by producing thrombocidins or by aggregating around the bacteria and “trapping” them for elimination by professional phagocytes. (B) It is now clear that platelets can also heterotypically interact with a wide variety of cells, including leukocytes. Activated platelets promote neutrophil tethering and activation through the expression of selectins, CD154 (also known as CD40L), and inflammatory cytokines and chemokines. (C) Similarly, activated platelets can promote the activation of monocytes and dendritic cells (DCs), particularly through CD40–CD154 interactions. This leads to increased antigen presentation to T cells and enhances adaptive immune responses. MHC, major histocompatibility complex; TCR, T-cell receptor. Reprinted from Semple et al,2 with permission from Macmillan Publishers Ltd.
Aside from their conventionally recognized function in hemostasis and thrombosis, platelets have received increasing attention for their roles in infectious diseases, innate immunity, and inflammation.2-4 To date, it has been unclear whether reduced platelet counts lead directly to adverse clinical outcomes in sepsis, or whether they are simply a biomarker for disease severity at presentation. An association between thrombocytopenia and mortality in septic shock patients was initially reported by Sharma et al in a study of 69 patients.5 In the larger study by Claushuis et al, which included 931 patients, the authors confirmed that mortality risk is elevated in sepsis patients with very low platelet counts upon admission to an intensive care unit. Although lower platelet counts were associated with higher Acute Physiology and Chronic Health Evaluation scores and more severe shock, platelet counts that were classified as very low (<50 × 109/L) or intermediate low (50 × 109/L to 99 × 109/L) were both associated with increased 30-day mortality, independent of disease severity.
The authors also observed that very low platelet counts (<50 × 109/L) were associated with elevated plasma levels of interleukin (IL)-8 and IL-10, elevated endothelial activation biomarkers (increased intercellular adhesion molecule 1 and fractalkine), reduced vascular integrity (increased ratio of angiopoietin [Ang]-2 to Ang-1), and increased coagulation activity (decreased antithrombin). These findings implicate several important host response pathways in the relationship between thrombocytopenia and adverse clinical outcomes. Notably, the associations between platelet counts and host response pathways remained statistically significant after rigorous adjustment using propensity matching to account for baseline differences in disease severity and other factors.
In support of a role for platelets in host response to sepsis, patients with thrombocytopenia had whole-blood leukocyte transcriptome patterns that revealed underexpression of genes encoding proteins involved in leukocyte adhesion, diapedesis, and extravasation signaling. Although these results do not demonstrate a causal association between platelet counts and host response, the findings are consistent with the preclinical animal studies reviewed by the authors in the manuscript’s discussion. The importance of these findings lies in their demonstration of the significance of thrombocytopenia as a risk factor for both dysregulated host response and adverse clinical outcomes.
Platelets can participate with immune cells in immune-driven thrombus formation and play a key role in the formation of neutrophil extracellular traps, which can aid in the capture and killing of bacteria and viruses.2,6,7 In addition, platelets can interact with bacterial pathogens to kill them directly via microbicidal proteins, known as thrombocidins (see figure panel A).2 Platelets also interact with immune cells, enhancing a number of immune functions via cytokine and chemokine secretion and promoting neutrophil tethering at sites of damaged endothelium (see figure panel B).2,3,7 Finally, activated platelets can increase antigen presentation to T cells, thereby enhancing the activation of adaptive immune responses (see figure panel C).2,3,7
Endothelial stabilization is another potential mechanism that could account for the effect of platelets on clinical outcomes in sepsis. Over the past 15 years, our understanding of sepsis has evolved beyond rogue inflammation and compensatory anti-inflammatory responses.
Sepsis has been increasingly recognized by a number of investigators as a syndrome of severe infection-related endothelial activation and dysfunction that leads to systemic microvascular leak and multiple-organ failure.8,9 The Ang-Tie2 system, in particular, has emerged as an important regulator of endothelial activation status.10 In general, Ang-1 stabilizes the endothelium and prevents microvascular leak, and its major sources are pericytes and platelets. Systemic serum or plasma levels of Ang-1 decline precipitously in severe sepsis, as reported previously and in the current study.1,10 Therefore, it seems entirely plausible that thrombocytopenia in sepsis could contribute to adverse clinical outcomes by decreasing the delivery of bioavailable Ang-1 to endothelium at risk.
Overall, Claushuis et al have advanced the field by highlighting the importance of platelets in a large cohort of critically ill patients with clinical sepsis. This study was limited by the inability to demonstrate causality inherent to observational clinical studies and by somewhat limited generalizability due to inclusion of only one clinical site in the Netherlands. Further study is needed to identify potential interventions that may be of use in patients with low platelet counts at baseline, using animal models initially. It is unclear whether platelet transfusions or specific interventions to enhance platelet immune function or other innate immune mechanisms would be of value. However, the findings reported by Claushius et al clearly suggest that the roles of platelets in innate immunity, inflammation, and endothelial stabilization may be as clinically important and multifaceted as their role in hemostasis and thrombosis.
Conflict-of-interest disclosure: W.C.L. is listed as co-inventor on a patent applied for by the University Health Network (Toronto, ON, Canada) to develop point-of-care tests for endothelial activation biomarkers in infectious diseases. S.M.G. declares no competing financial interests.