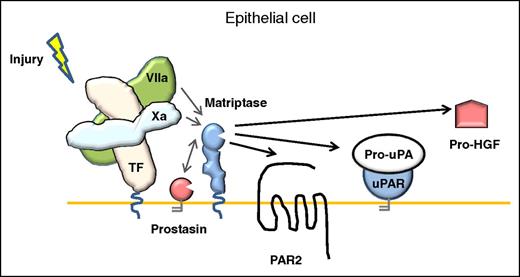
In this issue of Blood, Le Gall et al show that matriptase, a member of the type II transmembrane serine protease family, is a key coordinator of tissue factor (TF)–dependent activation of protease-activated receptor-2 (PAR2) on epithelial cell lines by coagulation proteases.1
Model for coagulation signaling to epithelia. In contrast to the activation mechanism in endothelial cells, the activation of PAR-2 signaling on the surface of epithelial cells mediated by TF:FVIIa, FXa, or the ternary TF:FVIIa:FXa complex, can be amplified by the membrane-anchored serine protease matriptase. uPAR, uPA receptor.
Model for coagulation signaling to epithelia. In contrast to the activation mechanism in endothelial cells, the activation of PAR-2 signaling on the surface of epithelial cells mediated by TF:FVIIa, FXa, or the ternary TF:FVIIa:FXa complex, can be amplified by the membrane-anchored serine protease matriptase. uPAR, uPA receptor.
An expanding body of literature points to an important role of coagulation protein-mediated cell signaling of vascular repair by the activation of PARs. PARs constitute a family of 4 seven-transmembrane G-protein coupled receptors (PAR1-4) that mediate cellular responses to serine proteases and may be processed by different activating or inactivating proteases.2 The PARs are activated by proteolytic cleavage, exposing a new amino-terminus that serves as a tethered ligand, initiating diverse intracellular signal transduction pathways. Although PAR1, PAR3, and PAR4 on platelets and endothelial cells are receptors for thrombin, PAR2 is a target of multiple trypsin-like serine proteases, including matriptase.3
In previous studies, Camerer et al4 demonstrated that PAR2 could be activated directly by TF/factor VIIa (FVIIa) and indirectly by TF/FVIIa-generated factor Xa (FXa) in keratinocytes and cytokine-treated endothelial cells, implicating for the first time a role for coagulation factors in PAR2 activation. To investigate this activity in more detail, Le Gall et al generated a PAR2G35K mutant, which was resistant to FVIIa and FXa cleavage but was shown to still be efficiently cleaved by matriptase. To the authors’ surprise, both FVIIa and FXa induced robust TF-dependent cleavage of PAR2G35K in 2 epithelial cells lines, whereas cells that did not express active matriptase did not give significant signals despite TF coexpression. In a series of detailed and well-controlled biochemical experiments using transfected cell lines and recombinant protease domains, the authors showed that FVIIa and FXa activate the matriptase zymogen on epithelial cells. The activated matriptase induced amplified signaling responses, including increased transepithelial electrical resistance, a measure of epithelial barrier function, as well as increased PAR2-dependent mitogenic extracellular signal-regulated kinase phosphorylation. The downstream effects of matriptase activation by the coagulation proteases was not only limited to PAR2 signaling, but also induced the activation of additional matriptase substrates, the fibrinolytic serine protease pro-urokinase plasminogen activator (pro-uPA) and the mitogenic growth factor pro-hepatocyte growth factor (pro-HGF) (see figure).
Where and how does this activation take place in vivo? Matriptase is expressed in virtually all epithelia, and is found localized along the basolateral surfaces and adherens junctions of simple polarized epithelia,5 where it plays a key role in epithelial homeostasis.5,6 Null and hypomorphic mutations in the matriptase gene ST14 are linked to defects in epidermal barrier formation in humans displaying inherited skin disease autosomal recessive ichthyosis with hypotrichosis. The recent generation of murine models of inducible matriptase ablation in the whole animal, and tissue-specific ablation in the gastrointestinal tract and salivary epithelium, has revealed that matriptase is essential for the maintenance of epithelial barrier integrity.6 Matriptase is synthesized in cells as an inactive zymogen. Its processing to the active form at the cell surface is currently poorly understood. Matriptase is able to be catalytically activated by the glycosylphosphatidylinositol-anchored serine protease, prostasin,7 and is also capable of auto-activation at the cell surface, an unusual mechanism among the serine proteases. Here, the authors report a new pathway for matriptase activation associated with tissue injury. Matriptase-mediated signaling might occur in vivo when the plasma proteins FVIIa and FXa contact TF-expressing keratinocytes and other epithelial cells at sites of wounding, inflammation, or altered vascular integrity.
It is becoming clear that matriptase activation and cellular responses are tissue-context specific and dependent on the extracellular milieu. Whether differential activation of matriptase by proteases elicits different protease-specific cell signaling responses is at present unclear. Membrane-anchored protease-dependent PAR2 cleavage may be expected to target PAR-signaling responses to specific cellular microdomains such as within lipid rafts, whereas soluble proteases would cleave PARs independent of location. Moreover, released FXa or trypsin may generate a response with high magnitude but limited duration, whereas activation of matriptase by membrane-targeted proteases could generate prolonged PAR2 signaling responses.
The link between matriptase and coagulation initiation could be expected to contribute to the pathogenic effects of extrinsic pathway activation in cancer and inflammation.8 Although vascular inflammation induced by TF is unlikely to involve matriptase because matriptase is not expressed by endothelial cells, bleeding or ectopic expression of FVIIa could trigger TF and matriptase-dependent pathologies that are both dependent and independent of PAR2 in epithelia. Tumor-expressed matriptase activation by coagulation proteases could contribute to facilitating the activation of pro-HGF, pro-uPA, and potentially other matriptase substrates alongside PAR2 and fibrin downstream of TF to promote tumor growth and dissemination.9,10
Future investigations of PAR2 activation by the TF-dependent FVII/Xa/matriptase pathway are warranted in order to extend these findings to human pathology.
Conflict-of-interest disclosure: The author declares no competing financial interests.