Key Points
HD-DXM is a preferred strategy to conventional prednisone as first-line management of newly diagnosed adult primary ITP.
Abstract
This study compared the efficacy and safety of high-dose dexamethasone (HD-DXM) and conventional prednisone (PDN) on the largest cohort to date as first-line strategies for newly diagnosed adult primary immune thrombocytopenia (ITP). Patients enrolled were randomized to receive DXM 40 mg/d for 4 days (n = 95, nonresponders received an additional 4-day course of DXM) or prednisone 1.0 mg/kg daily for 4 weeks and then tapered (n = 97). One or 2 courses of HD-DXM resulted in a higher incidence of overall initial response (82.1% vs 67.4%, P = .044) and complete response (50.5% vs 26.8%, P = .001) compared with prednisone. Time to response was shorter in the HD-DXM arm (P < .001), and a baseline bleeding score ≥8 was associated with a decreased likelihood of initial response. Sustained response was achieved by 40.0% of patients in the HD-DXM arm and 41.2% in the PDN arm (P = .884). Initial complete response was a positive indicator of sustained response, whereas presence of antiplatelet autoantibodies was a negative indicator. HD-DXM was generally tolerated better. We concluded that HD-DXM could be a preferred corticosteroid strategy for first-line management of adult primary ITP. This study is registered at www.clinicaltrials.gov as #NCT01356511.
Medscape Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint providership of Medscape, LLC and the American Society of Hematology.
Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 370.
Disclosures
The authors, Editor Nancy Berliner, and CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declare no competing financial interests.
Learning objectives
Compare the efficacy of high-dose dexamethasone vs conventional prednisone as a first-line strategy for newly diagnosed adult idiopathic thrombocytopenia, based on a prospective, multicenter, randomized trial.
Compare the safety of high-dose dexamethasone vs conventional prednisone as a first-line strategy for newly diagnosed adult idiopathic thrombocytopenia.
Describe other advantages of high-dose dexamethasone and predictors of response to this drug as a first-line strategy for newly diagnosed adult idiopathic thrombocytopenia.
Release date: January 21, 2016; Expiration date: January 21, 2017
Introduction
Immune thrombocytopenia (ITP) is an autoimmune thrombocytopenic syndrome characterized by decreased platelet count and an increased risk of bleeding. Conventionally, the pathogenesis of ITP has been described as autoantibody-mediated platelet overdestruction. Recent studies indicated more complex mechanisms including cytotoxic T-lymphocyte–induced platelet lysis, impaired megakaryocyte maturation, and insufficient platelet production. Immune abnormalities of T-lymphocyte subsets play essential roles in the cellular and molecular mechanisms of ITP.1 Corticosteroids are recommended as the first-line therapeutic strategy in practical guidelines. Patients failing first-line treatment should be managed with splenectomy or second-line agents (eg, thrombopoietic-stimulating agents, rituximab).2-4
Prednisone (PDN) is the standard initial treatment of ITP, usually given at 0.5 to 2 mg/kg bodyweight daily for 4 weeks to the maximum and then tapered.3 Initial response can be anticipated in about two-thirds of patients, but long-term remission is seen only in a limited fraction of patients. Moreover, adverse events caused by corticosteroids often outweigh the benefits because of the long-term administration.3-5 Pulsed high-dose dexamethasone (HD-DXM) was originally used for management of refractory ITP.6 Cheng et al introduced a single course of HD-DXM for treatment-naive ITP patients and achieved 85% initial response and 42% sustained response (SR) at 6 months’ follow-up.7 Another multicenter cohort study using repeated courses of HD-DXM also demonstrated encouraging results.8 These studies suggested that it is possible to shorten the duration and reduce the adverse effects of corticosteroid treatment. However, it must be recognized that neither of these studies included PDN as a control; therefore, a randomized comparison is still needed to clarify which of the 2 corticosteroid regimens is superior.3
As consensus of definitions and outcome criteria in ITP have been achieved,9 it is important to reassess the efficacy of ITP medications using the standardized criteria. With this prospective, randomized, controlled clinical trial, we aimed to compare the efficacy and safety of HD-DXM and PDN as first-line strategies for newly diagnosed adult primary ITP.
Methods
Study design
This was a prospective, multicenter, randomized, controlled, open-label clinical trial comparing HD-DXM and PDN for first-line management of newly diagnosed adult ITP. The study was conducted in collaboration among 9 separate investigation sites in China. Data were collected from each participating site and sent to the principal investigation site at Qilu Hospital, Shandong University, for analysis. The study protocol was approved by the ethics committee on medical research of each participating site. All patients provided written informed consent in accordance with the Declaration of Helsinki before enrollment.
Patients
Patients eligible for enrollment were aged 18 years or older of both genders and were diagnosed with primary ITP according to the International Working Group (IWG) guidelines.3 Bone marrow examination was required to confirm the diagnosis of ITP for patients older than age 60 years3 or those with abnormalities in peripheral blood cells other than thrombocytopenia. All patients enrolled had newly diagnosed,9 treatment-naive ITP, with either a baseline peripheral platelet count <30 × 109/L or with the presence of bleeding symptoms at enrollment. However, for safety considerations, patients with life-threatening bleeding (eg, massive hemorrhage with severe anemia, central nervous system bleeding) were not permitted to enroll.
Any previous ITP-specific therapy was considered an exclusion criterion. Patients who had received corticosteroids or immunosuppression therapy for non-ITP diseases within 3 months before enrollment were excluded. Other exclusion criteria included malignancy, connective tissue diseases, seropositive detection of HIV, hepatitis B virus or hepatitis C virus, pregnancy or lactation, active infection, hypertension, diabetes, cardiovascular diseases, liver and kidney function impairment, psychosis, and osteoporosis.
Procedures
Eligible patients were randomly assigned 1:1 to receive either HD-DXM (the HD-DXM arm) or conventional-dose PDN (the PDN arm). Randomization was conducted centrally at Qilu Hospital, Shandong University, using precoded concealed envelopes generated by permuted-block randomization with a block size of 4. No blinding of physicians or patients was used. Bleeding symptoms were graded by a standardized ITP-specific bleeding scale based on the site and severity of bleeding.10 The bleeding score was calculated by adding the points relevant to various clinical bleeding signs. A modification was made to exclude age from the original scale so that only bleeding symptoms were described.
In the HD-DXM arm, DXM was administered orally at 40 mg daily for 4 consecutive days and then stopped. If platelet count remained below 30 × 109/L or there were bleeding symptoms by day 10,7 an additional 4-day course of DXM (40 mg daily) was given. Because platelet counts should be confirmed on 2 separate occasions at least 7 days apart when defining response,9 the additional course of medication was also administered to patients who transiently fulfilled criteria of response but did not show response at the time of confirmation. Patients in the PDN arm received PDN orally at 1.0 mg/kg body weight daily for 4 consecutive weeks. In responders, the medication was tapered gradually to a maintenance dose of less than 15 mg daily or complete termination. The taper schedule was determined by physicians but was supposed to be within 4 to 6 weeks. Continued low doses were aimed at maintaining a platelet count above 30 × 109/L with an absence of bleeding symptoms. If complete response (CR) was achieved, the taper could begin as early as the third week. PDN was rapidly tapered to termination in nonresponders after 4 weeks. In both arms, nonresponders exited the study and other treatments were considered. Platelet transfusion and hemostatic agents were permitted to prevent severe bleeding and were recorded. Requirements for any additional ITP-modifying intervention were considered as treatment failure.
To identify the prognostic values of antiplatelet autoantibodies in ITP, baseline anti–GPIIb-IIIa and anti–GPIb-IX autoantibodies were tested in all patients by a modified monoclonal antibody-specific immobilization of platelet antigens, assay as previously described by Hou et al.11
Outcomes measures
The primary end points were initial response and SR. CR was defined as platelet count ≥100 × 109/L and absence of bleeding; response (R) as platelet count ≥30 × 109/L and at least 2-fold increase of the baseline count and absence of bleeding; no response as platelet count <30 × 109/L or less than 2-fold increase of the baseline platelet count or bleeding. Platelet counts should be confirmed on 2 separate occasions at least 7 days apart when defining CR or R.9 Initial responses were assessed by day 10 in the HD-DXM arm7 and day 28 in the PDN arm, respectively. We defined SR as platelet count maintained >30 × 109/L with an absence of bleeding symptoms or no requirement for additional ITP-modifying treatment of 6 consecutive months following achievement of initial response.7,12
Secondary end points included bleeding scores, time to response, duration of response, and adverse events. Bleeding scores were assessed at both baseline and evaluation of the initial response. Time to response was defined as the duration from initiation of treatment to achievement of CR or R. Duration of response was measured from the achievement of CR or R to loss of response (platelet count dropped below 30 × 109/L or presence of bleeding) or to the last follow-up visit. Initial responders underwent monthly follow-up visits for at least 1 year or until loss of R. Adverse events were evaluated according to Common Terminology Criteria for Adverse Events, version 3.0, published by US National Cancer Institute. Patients who experienced severe adverse effects would stop the allocated intervention and exit the study by determination of physicians. Each patient underwent a safety follow-up for 1 month after medication was terminated.
Statistical analysis
Calculation of sample size was based on 1 of the primary end points, the initial response, resulting from the lack of definite data for SR. It was estimated that 85% of patients would respond to 1 or 2 courses of HD-DXM and 65% to conventional PDN. Therefore, 81 evaluable cases per arm were necessary in order to detect a difference in the incidence of initial response with 90% power of test at 5% significance level (by 2-sided Fisher’s exact test). We also set up a 20% redundancy to balance possible dropout or other bias and the sample size was finalized to be 97 cases per arm.
We included all patients who received allocated intervention as intention-to-treat population in the description of baseline characteristics and the analysis of efficacy and safety. To provide supplemental data, incidences of the 2 primary end points were described by per-protocol (PP) population, which excluded cases discontinuing the allocated intervention and cases lost to follow-up. Descriptive statistics were used to summarize baseline characteristics and safety data. Incidences of initial and SR were compared between the 2 arms by Fisher’s exact test. We used a logistic regression model to evaluate the correlation between certain baseline parameters and initial or sustained response. The odds ratio (OR) was provided and estimated together with 95% confidence interval (CI). The Kaplan-Meier method and log-rank test were used to evaluate differences in duration of response between groups. Baseline parameters, bleeding scores, time to response and platelet counts at follow-up visits were compared between the 2 arms with appropriate statistical tests (eg, Fisher’s exact test, dependent sample t test). Analyses were conducted using IBM SPSS Statistics, version 19. All tests were 2-sided at a significance level of 5%.
Results
Characteristics
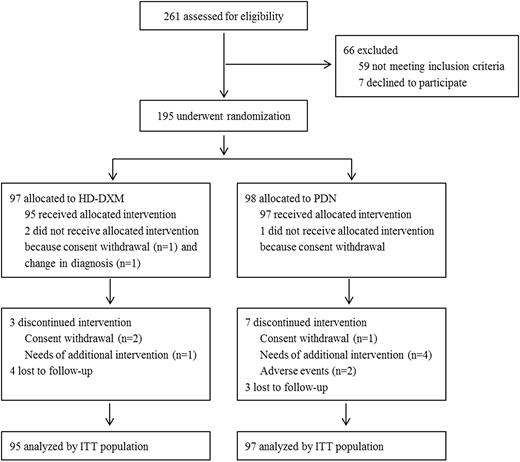
Between January 2011 and May 2014, 261 patients were screened for eligibility and 195 were ultimately enrolled, of whom 97 were randomly assigned to the HD-DXM arm and 98 to the PDN arm. Two cases in the HD-DXM arm and 1 in the PDN arm did not receive the allocated intervention due to consent withdrawal (1 vs 1) or amended diagnosis (1 vs 0). Three cases in the HD-DXM arm and 7 in the PDN arm exited the study during treatment due to consent withdrawal (2 vs 1), requirement of additional intervention (1 vs 4) or adverse events (0 vs 2). Four cases in the HD-DXM arm and 3 in the PDN arm were lost to follow-up before the evaluation of SR (Figure 1).
Baseline characteristics were comparable between the 2 arms (Table 1). There were more females than males with no significant difference in age between genders. One hundred and 55 patients (80.2%) across the 2 arms presented with bleeding symptoms, generally mild to moderate with a median grade of 4 (range, 0-13). Skin (64.1%) and mucus (32.8%) were the most common bleeding sites. Visceral bleeding was observed in 26 patients (13.5%) and no intracranial bleeding was recorded. One patient in the PDN arm with a baseline platelet count of 36 × 109/L was enrolled for gingival bleeding. The baseline platelet count was 10 × 109/L or less in 116 patients (60.4%). These patients presented with significantly more severe bleeding manifestations, with a median bleeding score of 4.5 (range, 0-12) vs 3 (range, 0-13) in patients with a baseline platelet count over 10 × 109/L (P < .001).
Initial response
As shown in Table 2, 1 or 2 courses of HD-DXM resulted in a higher incidence of overall response compared with PDN. However, the difference was not significant if evaluated by PP population (84.8% vs 74.4%, P = .099). HD-DXM also resulted in a higher incidence of CR and a shorter time to response (Table 2). In the HD-DXM arm, 67.4% of patients responded to the initial course of medication, with a CR rate of 45.3%. Twenty-eight patients received an additional 4-day course of HD-DXM; of these, half responded and 5 achieved CR. The additional course of medication significantly increased the incidence of overall response in this arm (P = .029), although patients responding to the additional course were less likely to achieve CR than those who responded to the initial course (P = .037). Bleeding was more effectively controlled in the HD-DXM arm, with fewer bleeding events (12 vs 25, P = .028) and lower bleeding scores (P = .030). There was no significant difference in the bleeding scores of nonresponders compared with their baseline levels in both arms (P = .078 in the HD-DXM arm; P = .391 in the PDN arm).
We evaluated potential prognostic parameters under the logistic regression model. In both arms, a baseline bleeding score ≥8 had a negative impact on the initial response (OR, 0.253; 95% CI, 0.069-0.922 in the HD-DXM arm, and OR, 0.210; 95% CI, 0.062-0.706 in the PDN arm). Gender, age, and baseline platelet count had no significant correlation with response to either corticosteroid regimen.
Long-term outcomes
The incidence of SR and sustained CR showed no significant difference between the 2 arms (Table 2). There was also no significant difference in the incidence of SR evaluated by PP population (43.2% vs 46.0%, P = .762). Patients who responded to the additional course of HD-DXM were as likely to achieve SR as those responding to the initial course (P = .141). Initial CR was the only definite positive indicator associated with an increased incidence of SR in the initial responders of both arms analyzed by the logistic regression model (OR, 0.178; 95% CI, 0.062-0.514 in the HD-DXM arm, and OR, 0.251; 95% CI, 0.071-0.885 in the PDN arm). Moreover, none of the baseline parameters was significantly correlated with SR in either arm.
Duration of PDN administration is shown in Table 3. Approximately 60% of the patients received PDN for less than 3 months, and 8 (8.2%) patients stayed on PDN for more than 1 year. We attempted to compare the incidence of SR without maintaining therapy between the 2 arms. Because a 3-month interval might be necessary to exclude the impact of the maintaining dose of PDN, the comparison was made between the HD-DXM arm and the 25 patients (15 achieving SR) who were able to terminate PDN within 3 months of follow-up. These patients presented comparable incidence of SR (P = .365).
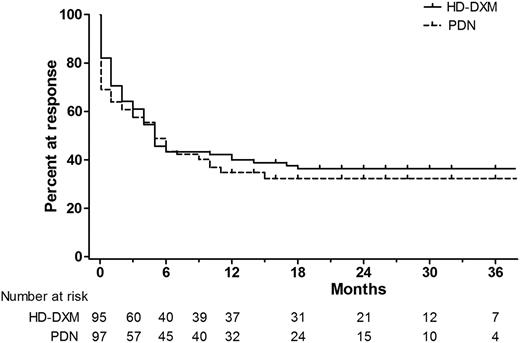
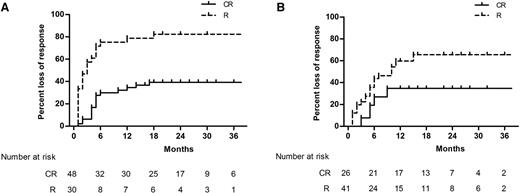
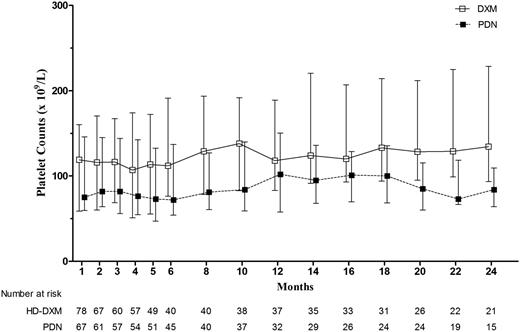
At 12 months’ follow-up, a comparable fraction of patients remained in response, with 36.8% of patients in the HD-DXM arm vs 33.0% in the PDN arm (P = .650). Throughout the follow-up period, overall duration of response was similar between the 2 arms, estimated by the Kaplan-Meier analysis (Figure 2; P = .522). We further estimated the duration of response stratified by the quality of the initial response (CR or R). The Kaplan-Meier cumulative incidence of loss of response was significantly lower among initial CR responders in both arms (Figure 3; P < .001 in the HD-DXM arm; P = .022 in the PDN arm). During months 1 to 24 of follow-up, median platelet counts of patients in the HD-DXM arm ranged from 107 to 138 × 109/L and from 72 to 102 × 109/L in the PDN arm (Figure 4). Platelet counts were compared between the 2 arms at various time points during the follow-up period, and the HD-DXM arm represented higher mean platelet counts at most time points (P < .05). Patients in the HD-DXM arm benefitted from consistently higher platelet levels subsequent to the achievement of initial response.
Kaplan-Meier estimates of the duration of response. The Kaplan-Meier curve demonstrated comparable long-term outcomes between the 2 arms (P = .522).
Kaplan-Meier estimates of the duration of response. The Kaplan-Meier curve demonstrated comparable long-term outcomes between the 2 arms (P = .522).
Kaplan-Meier estimates of the cumulative loss of response stratified by initial response quality (CR or R). (A) Incidence of loss of response was significantly lower among initial CR responders in the HD-DXM arm (P < .001). (B) Incidence of loss of response was significantly lower among initial CR responders in the PDN arm (P = .022).
Kaplan-Meier estimates of the cumulative loss of response stratified by initial response quality (CR or R). (A) Incidence of loss of response was significantly lower among initial CR responders in the HD-DXM arm (P < .001). (B) Incidence of loss of response was significantly lower among initial CR responders in the PDN arm (P = .022).
Median platelet counts (×109/L) with interquartile range of initial responders at follow-up visits.
Median platelet counts (×109/L) with interquartile range of initial responders at follow-up visits.
Antiplatelet autoantibodies
Antiplatelet autoantibodies were detected in 102 patients (53.1%, Table 1). Presence of anti–GPIIb-IIIa autoantibody was associated with both lower baseline platelet count (P = .028) and more severe bleeding manifestations (P = .046). In both arms, neither of the 2 autoantibodies was correlated with the incidence of initial response (P > .05). However, any presence of antiplatelet autoantibodies (including single and double positive) significantly indicated reduced opportunity to achieve SR in both arms (P = .021 in the HD-DXM arm; P = .024 in the PDN arm).
Safety
No deaths were recorded throughout the treatment or the follow-up periods. Adverse events are summarized in Table 4, and patients ≥60 years of age are listed separately. Both treatments were well tolerated in general. Most of the adverse effects were mild to moderate (graded 1 or 2) and usually resolved spontaneously after medication was completed. All adverse events had been previously described or reported. The frequency of adverse events was higher in the PDN arm, especially with cushingoid appearance and weight gain in >10% of the patients. Several other adverse events were observed in >5% of the patients in each arm, including insomnia and mood disorders in the HD-DXM arm, and dizziness, hyperglycemia, hypertension, insomnia, and peptic ulcer in the PDN arm.
Of the 2 patients who discontinued treatment because of adverse effects in the PDN arm, 1 was a 74-year-old woman who suffered from pneumonia and required intravenous antibiotics to control the infection; the other was a 59-year-old woman with grade 3 hyperglycemia and no history of diabetes mellitus or impaired glucose tolerance. Neither patient showed an elevation in platelet count by the time of exit. No patients exited the study because of adverse effects in the HD-DXM arm.
Discussion
Although guidelines by the American Society of Hematology recommended longer courses of corticosteroids as first-line treatment,2 it should be noted that this recommendation was based on a randomized, controlled trial (RCT) in which a short course of high-dose methylprednisolone followed by PDN acted more effectively than that followed by placebo.13 Retrospective studies comparing the 2 approaches in smaller cohorts yielded conflicting results14-16 ; thus, RCTs directly comparing these 2 corticosteroid regimens are required to provide higher level evidence of efficacy. The design of this study was based on the standardized definitions and outcome criteria of the IWG.9 Treatment-naive patients were studied to avoid possible bias in baseline characteristics with respect to previous therapies.
The HD-DXM arm was designed to begin with a single 4-day course of HD-DXM based on results from Cheng et al.7 We made a modification to allow the administration of an additional 4-day course of medication to patients who failed to respond to the initial course. After the initial single course, response in our study did not reach the 85% level observed by Cheng et al,7 but did resemble the data after 1 course of HD-DXM by Mazzucconi et al (69.5%).8 Patients who failed to respond to the initial course still could respond to the additional course of medication. Data obtained from the DXM group of 2 randomized controlled studies revealed similar incidences of SR (36% and 37%, respectively)12,17 compared with Cheng et al (42%).7 Our results of SR are consistent with the findings from those studies.
Numerous studies, mostly retrospective, had presented data on the standard dose of PDN or its equivalent for initial treatment of adult primary ITP. In most studies, initial response was achieved in approximately two-thirds of patients.3-5 Nevertheless, long-term outcomes are difficult to compare among studies because of the various definitions of outcomes and duration of follow-up.3-5 The incidence of initial response in this study was in accordance with previously reported studies under similar PDN administration routines.18-21 Because a limited dose of PDN was permitted to sustain a safe platelet level in our design, long-term outcomes in the PDN arm could be attributed to this maintenance therapy especially in a proportion of initial “R” responders.
Our results suggest that 1 or 2 courses of HD-DXM worked more effectively and rapidly than conventional PDN, and resulted in an outstanding incidence of CR. The incidence of overall response was higher in the HD-DXM arm, though the difference was not statistically significant by PP population. HD-DXM also more significantly reduced bleeding manifestations. Both the average bleeding score and the number of patients presenting bleeding symptoms were lower in the HD-DXM arm. These 2 interventions led to comparable SR rates and duration of response by Kaplan-Meier analysis. We found that initial CR was a definite positive predictor of better long-term outcomes. However, patients in the HD-DXM arm generally benefitted from higher platelet level subsequent to achieving initial response. These findings suggest that HD-DXM could be a more effective choice at the early stage of treatment and could produce at least equivalent long-term outcomes to conventional PDN without the burden of long-term corticosteroid administration.
No deaths occurred throughout our study. One reason was that, for safety, we excluded patients with life-threatening bleeding at enrollment. Most adverse effects were tolerable. Occurrence of adverse events was more frequent and long-lasting in the PDN arm, with 2 cases discontinuing therapy because of adverse effects. The better tolerance of HD-DXM might be attributed to its limited duration because adverse events in this arm were usually transient and spontaneously resolved after the completion of medication. The treatments were also well tolerated in patients ≥60 years of age. HD-DXM allowed avoidance of the safety risks associated with the long-lasting administration of PDN.
In addition to platelet count, bleeding manifestations should also be considered as an essential parameter of disease severity in ITP.3,9 We chose an ITP-specific bleeding scale10 rather than the World Health Organization scale22 (focused mainly on the amount of blood loss), because this scale was able to reflect the severity and sites of bleeding that were considered more meaningful in ITP. The IWG has published a standardization of bleeding assessment in ITP based on bleeding manifestations and severity23 ; however, unfortunately, it was not yet available when our study was designed in 2010. Under the selected scale, patients with a baseline platelet count of 10 × 109/L or lower were associated with more severe bleeding. We also found that a baseline bleeding score ≥8 was a negative predictor of initial response in corticosteroid strategy.
Antiplatelet autoantibodies were detected in approximately half of the enrolled patients, comparable to a recent observational registry study.24 Though not recommended as a diagnostic parameter in practice guidelines,2,3 a test of antiplatelet autoantibodies showed certain prognostic potentials according to data from our study and others.25,26
In conclusion, results from this prospective, multicenter, randomized, controlled study suggest that 1 or 2 courses of HD-DXM provides a more effective and more rapid response as initial treatment of ITP, with at least comparable long-term prognosis and better tolerance when compared with conventional PDN. HD-DXM also enables patients to avoid the burden of long-term corticosteroids. Therefore, HD-DXM could become a preferred corticosteroid approach for first-line management of adult primary ITP. Furthermore, because it has been shown that repeated courses of medication may yield better long-term outcome,8 future RCTs should be designed to compare the effect of repeated courses vs a limited course of HD-DXM.
Presented in part by poster at the 56th annual meeting of the American Society of Hematology, San Francisco, CA, December 6-9, 2014.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Yu Hou from the Department of Pathology, School of Medicine, Stanford University, and Dr Alexandra Marshall from the St. Michael's Hospital, University of Toronto, for language editing of the manuscript.
This work was supported by grants from Taishan Scholar of Shandong Province, National Natural Science Foundation of China (81200344, 81270578, 81300383, 81370623, 81370616, 81300384, 81300382, 81470284, 81470285), National Natural Science Foundation for Distinguished Young Scholars of China (81125002), the Major Research plan of the National Natural Science Foundation of China (91442204), State Program of National Natural Science Foundation of China for Innovative Research Group (81321061), National Key Basic Research Program of China (973 Program, 2011CB503906), National High Technology Research and Development Program of China (863 Program, 2012AA02A505), State Key Clinical Specialty of China for Blood Disorders, National Public Health Grand Research Foundation (201202017), Shandong Province Science and Technology Development Project (2014GSF118035, 2014GSF118036), the Natural Science Foundation of Shandong Province (ZR2013HQ001), the Fundamental Research Funds of Shandong University (2015JC013, 2015QLMS07), and the Independent Innovation Foundation of Shandong University (2012TS168).
Authorship
Contribution: Y.W., X.-b.J., Y.-w.W., J.P., and M.H. designed and performed research, analyzed data, and wrote the paper; J.-x.W., E.-q.Y., Z.-c.W., Y.-q.S., and Z.-m.B. performed research and analyzed data; C.-a.R., F.Z., G.-q.L. performed research and corrected the paper. All authors read and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ming Hou, Department of Hematology, Qilu Hospital, Shandong University, 107 Wenhuaxi Rd, Jinan 250012, China; e-mail: qlhouming@sina.com; and Jun Peng, Key Laboratory of Cardiovascular Remodeling and Function Research, Chinese Ministry of Education and Chinese Ministry of Health, Qilu Hospital, Shandong University, 107 Wenhuaxi Rd, Jinan 250012, China; e-mail: junpeng88@sina.com.cn.
References
Author notes
Y.W., X.-b.J., and Y.-w.W. contributed equally to this work.