Key Points
CD20 Bdep therapy inhibits CD8+ T-cell proliferation in vitro.
CD20 Bdep therapy prevents CD8+ T-cell–mediated ITP in vivo.
Abstract
Immune thrombocytopenia (ITP) is an autoimmune bleeding disorder with a complex pathogenesis, which includes both antibody- and T-cell–mediated effector mechanisms. Rituximab (an anti-human CD20 monoclonal antibody [mAb]) is one of the treatments for ITP and is known to deplete B cells but may also work by affecting the T-cell compartments. Here, we investigated the outcome of B-cell depletion (Bdep) therapy on CD8+ T-cell–mediated ITP using a murine model. CD61 knockout (KO) mice were immunized with CD61+ platelets, and T-cell–mediated ITP was initiated by transfer of their splenocytes into severe combined immunodeficiency (SCID) mice. The CD61 KO mice were administrated an anti-mouse CD20 mAb either before or after CD61+ platelet immunization. This resulted in efficient Bdep in vivo, accompanied by significant increases in splenic and lymph node CD4+ and CD8+ T cells and proportional increases of FOXP3+ in CD4+and CD8+ T cells. Moreover, Bdep therapy resulted in significantly decreased splenic CD8+ T-cell proliferation in vitro that could be rescued by interleukin-2. This correlated with normalization of in vivo platelet counts in the transferred SCID mice suggesting that anti-CD20 therapy significantly reduces the ability of CD8+ T cells to activate and mediate ITP.
Introduction
Immune thrombocytopenia (ITP) is an autoimmune bleeding disorder characterized by an isolated thrombocytopenia (<100 × 109/L).1 The pathogenesis of chronic ITP is incompletely understood and thought to be heterogeneous.1 For example, approximately two-thirds of patients with ITP have detectable antiplatelet antibodies that destroy platelets primarily by Fc-mediated phagocytosis within the spleen.2,3 On the other hand, patients with no detectable antibodies can harbor platelet- and megakaryocyte-specific T cells that can mediate ITP.4,5 These effector responses are closely correlated with dysfunctional CD4+ T-regulatory cells suggesting T cells are critical for ITP development.6-8
B-cell depletion (Bdep) therapy using rituximab has been shown to be a successful second-line treatment of patients with ITP.9 Although B cells function to primarily elicit humoral immunity, they also have other roles such as antigen presentation, costimulation, and T-cell activation/cytokine production.10,11 Moreover, patients with ITP, whether antiplatelet antibody positive or not, can respond to rituximab and in those antibody-positive patients, their autoantibody titers do not necessarily change.12 This suggests that anti-CD20 can significantly affect T-cell compartments and this was originally confirmed by Stasi et al showing that rituximab normalizes the observed CD4+ T-cell abnormalities in ITP.6,13 To better understand how anti-CD20 therapy affects CD8+ T cells, we investigated its effect on T-cell–mediated thrombocytopenia in an established murine model of ITP.14 The data suggests that anti-CD20 Bdep significantly inhibits interleukin (IL)-2 dependent CD8+ T-cell proliferation, which blocks their ability to mediate ITP.
Study design
Mice
BALB/c mice (BALB/cAnNCrl, H-2d, CD61+; Charles River Laboratories, Montreal, QC, Canada) were used as platelet donors, and BALB/c CD61 (GPIIIa) knockout (KO) mice were supplied by Dr Heyu Ni and used as a source of immune splenocytes. CB17 severe combined immunodeficiency (SCID) mice (CB17/Icr-Prkdcscid/IcrIcoCrl, H-2d, CD61+; Charles River Laboratories) were used as splenocyte transfer recipients for induction of ITP. All mice were 8- to 12-weeks of age and all studies were approved by the St. Michael’s Hospital Animal Care Committee.
Bdep in a murine model of ITP
A murine ITP model was used as previously described.14 CD61 KO mice were immunized against CD61+ BALB/c platelets and their nondepleted (ND) splenocytes were transferred into SCID mice to initiate ITP.14 Splenocytes from some CD61 KO mice were depleted of CD19+ B cells in vitro before transfer to initiate CD8+ T-cell–mediated ITP (Bdep in vitro). Other KO mice were B-cell depleted (Bdep) in vivo by 2 IV injections of an anti-mouse CD20 monoclonal immunoglobulin G2a antibody (250 μg/mouse; Biogen, Cambridge, MA) either before or after platelet immunization to mimic Bdep therapy (Bdep pre or post, respectively). Details of the in vitro experimentation and the experimental design are shown in the supplemental Methods on the Blood Web site.
Statistical analysis
Data are expressed as mean ± standard deviation, and were analyzed using GraphPad Prism 6.02 software for Windows (GraphPad Software, San Diego, CA).
Results and discussion
Anti-CD20 antibody efficiently depletes B cells and increases the T-cell percentages in vivo
Murine monoclonal anti-CD20 antibodies derived from immunization of CD20 KO mice were first reported by Uchida et al and induce significant Bdep in vivo.15,16 In our hands, all the anti-CD20 antibody-treated mice were significantly depleted of B cells in the peripheral blood, spleen, and mesenteric lymph nodes (supplemental Figure 2A-B). In CD61 KO mice, Bdep before platelet immunization, a significant reduction in immunoglobulin G antiplatelet antibody production was also observed (supplemental Figure 2C-D). Bdep therapy in immune mice significantly increased the percentages of peripheral blood, splenic and lymph node CD3+ CD4+, CD3+ CD8+ T cells, and FOXP3+ T-regulatory cell subpopulations proportionally (supplemental Figures 3 and 4), consistent with previous studies showing that Bdep therapy increases the proportion of non–B-cell populations within immune compartments.17
Bdep therapy suppresses CD8+ T-cell proliferation in vitro
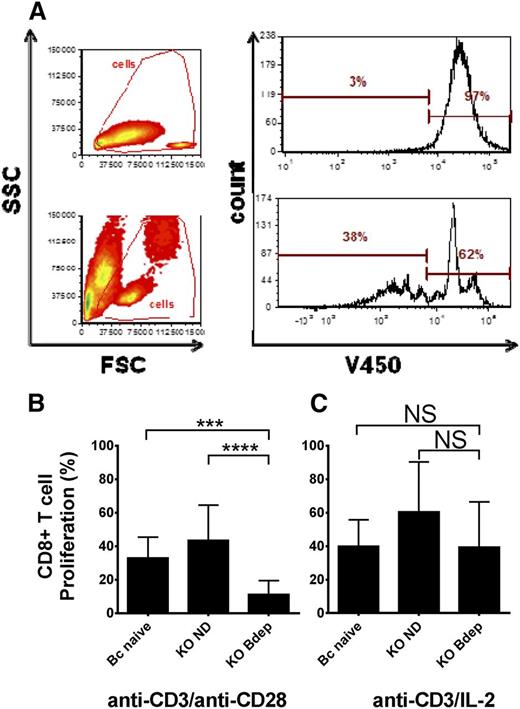
B cells have been previously shown to be involved in CD8+ T-cell maintenance and memory cell formation, and patients with ITP treated with rituximab have significant alterations of CD8+ T cells, including increased cytotoxicity of splenic CD8+ T cells in rituximab nonresponders.13,18-21 Therefore, we examined the proliferation potential of splenic CD8+ T cells from the ND or Bdep immune CD61 KO mice. Purified splenic CD8+ T cells were stained with V450 (VPD 450; BD Biosciences, Mississauga, ON) and cultured with anti-CD3 antibody together with either anti-CD28 antibody and/or recombinant IL-2 for 72 hours and the fluorescence intensity per cell was analyzed (Figure 1A). Compared with CD8+ T cells from either naïve BALB/c or ND CD61 immune KO mice, CD8+ T cells from the Bdep KO mice showed a significantly reduced proliferation upon anti-CD3 and anti-CD28 stimulation (Figure 1B). The deficient CD8+ T-cell proliferation could be rescued by the addition of recombinant IL-2 to the cultures (Figure 1C). Further examination of the splenic CD8+ T cells revealed increased intracellular Granzyme B expression after Bdep therapy (supplemental Figure 5). This may be related to how CD20low 4-1BBL+ B-regulatory cells regulate Granzyme B expression in CD8+ T cells.22,23 Although Granzyme B expression in CD8+ T cells was found to be increased in follicular lymphoma patients at diagnosis, which was associated with better prognosis, it appeared unchanged in patients with ITP.21,24 Taken together, our results suggest that in vivo Bdep therapy interferes with in vitro IL-2–dependent CD8+ T-cell activation but more research is required to characterize how this phenomenon occurs.
Bdep inhibits CD8+ T-cell proliferation in vitro. CD8+ T cells were purified from the spleens of BALB/c naïve mice (Bc naïve), platelet immunized CD61 KO mice (KO ND), and platelet immunized CD61 KO mice that received Bdep therapy during immunization (KO Bdep), and stained with the proliferation dye V450 and cultured in vitro with anti-CD3 ± anti-CD28/IL-2 for 72 hours. (A) Representative flow cytometic dot plot analysis of CD8+ T-cell proliferation when either not stimulated (top panels) or stimulated with anti-CD3/CD28 antibodies for 72 hours. A cell-division cycle is characterized by sequential halving of the V450 fluorescence. Cumulative data of (B) CD8+ splenic T cells stimulated with anti-CD3/anti-CD28 and (C) anti-CD3/IL-2. Data in (B-C) are expressed as the mean ± standard deviation of percent CD8+ T cells proliferating (n = 5-8 mice per group). Data were analyzed using one-way analysis of variance with a Tukey’s post hoc test (***P < .001; ****P < .0001). FSC, forward scatter; ns, nonsignificant; SSC, side scatter.
Bdep inhibits CD8+ T-cell proliferation in vitro. CD8+ T cells were purified from the spleens of BALB/c naïve mice (Bc naïve), platelet immunized CD61 KO mice (KO ND), and platelet immunized CD61 KO mice that received Bdep therapy during immunization (KO Bdep), and stained with the proliferation dye V450 and cultured in vitro with anti-CD3 ± anti-CD28/IL-2 for 72 hours. (A) Representative flow cytometic dot plot analysis of CD8+ T-cell proliferation when either not stimulated (top panels) or stimulated with anti-CD3/CD28 antibodies for 72 hours. A cell-division cycle is characterized by sequential halving of the V450 fluorescence. Cumulative data of (B) CD8+ splenic T cells stimulated with anti-CD3/anti-CD28 and (C) anti-CD3/IL-2. Data in (B-C) are expressed as the mean ± standard deviation of percent CD8+ T cells proliferating (n = 5-8 mice per group). Data were analyzed using one-way analysis of variance with a Tukey’s post hoc test (***P < .001; ****P < .0001). FSC, forward scatter; ns, nonsignificant; SSC, side scatter.
Bdep therapy induces normalization of platelet counts in a murine model of ITP
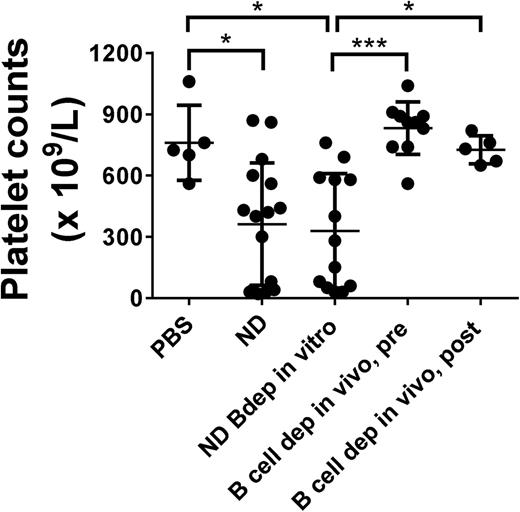
We further examined the effect of Bdep therapy on the development of ITP. SCID mice were transferred with splenocytes from immune CD61 KO mice that were either ND or depleted of CD19+ B cells in vitro to initiate antibody- and T-cell–mediated ITP, respectively. As previously described,14 both splenocyte populations induced significant thrombocytopenia when transferred into SCID mice (Figure 2, columns 1-3). In contrast, however, if the CD61 KO mice were Bdep in vivo either before or after platelet immunization, the ability of their splenocytes to induce ITP was completely prevented (Figure 2, columns 4-5).
In vivo Bdep results in a normalization of platelet counts in a murine model of T-cell–mediated ITP. Platelet counts in transferred recipient SCID mice after 21 days post-engraftment of 3 × 104 splenocytes. SCID mice were either transferred with phosphate-buffered saline or with ND splenocytes from platelet-immunized CD61 KO mice (ND), splenocytes from ND KO mice but depleted of CD19+ B cells in vitro (ND Bdep in vitro), or splenocytes from platelet-immunized CD61 KO mice depleted in vivo with anti-CD20 antibody before platelet immunization (Bdep in vivo, pre), or after platelet immunization (Bdep in vivo, post). Each data point represents 1 SCID mouse. Data were analyzed using one-way analysis of variance with a Tukey’s posttest (*P < .05; ***P < .001).
In vivo Bdep results in a normalization of platelet counts in a murine model of T-cell–mediated ITP. Platelet counts in transferred recipient SCID mice after 21 days post-engraftment of 3 × 104 splenocytes. SCID mice were either transferred with phosphate-buffered saline or with ND splenocytes from platelet-immunized CD61 KO mice (ND), splenocytes from ND KO mice but depleted of CD19+ B cells in vitro (ND Bdep in vitro), or splenocytes from platelet-immunized CD61 KO mice depleted in vivo with anti-CD20 antibody before platelet immunization (Bdep in vivo, pre), or after platelet immunization (Bdep in vivo, post). Each data point represents 1 SCID mouse. Data were analyzed using one-way analysis of variance with a Tukey’s posttest (*P < .05; ***P < .001).
Mechanistically, Bdep therapy in vivo may actively suppress or exhaust the proliferative potential of pathogenic CD8+ T cells upon activation and thereby limit their ability to induce ITP. Support for this possibility comes from studies showing that T cells, in the absence of B cells, have proliferation and memory development defects.18-20 In addition, although Bdep in vivo may modulate the balance between pro- and anti-inflammatory T-cell–derived cytokines,13 we did not observe any significant differences in interferon-γ production by CD8+ T cells (supplemental Figure 5) or intracellular expression of interferon-γ/IL-4 in CD4+ T cells (N = 5-8, data not shown) after Bdep therapy. Furthermore, in accordance with previous studies, the activation marker CD44 was not increased on the CD8+ T cells after Bdep therapy (supplemental Figure 5).18,19,25 Of interest, the interruption of B- and T-cell interactions by, for example, CD40L antibody, shows a similar therapeutic effect as rituximab in ITP, suggesting that a direct interaction between B cells and T cells is essential for ITP induction.12
In conclusion, our study suggests that the effectiveness of anti-CD20 therapy is due to induction of a significant CD8+ T-cell activation/proliferation defect via IL-2 blockade that correlates with their inability to induce thrombocytopenia. This may provide an additional explanation for the therapeutic effects of rituximab in T-cell–mediated ITP.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Gerald Prud’homme (St. Michael’s Hospital) for his helpful discussions.
This study was supported by grants from Health Canada and Canadian Blood Services to J.W.S. L.G. is the recipient of an Ontario Trillium Scholarship and a China Scholarship Council Scholarship. R.K. is the recipient of a postdoctoral fellowship from the Canadian Blood Services. A.Z. is the recipient of a postdoctoral fellowship from the Swiss National Science Foundation (P2GEP3_151966).
Authorship
Contribution: L.G. designed research, performed experiments, collected data, analyzed and interpreted data, performed statistical analysis, and wrote the manuscript first draft; R.K. analyzed and interpreted data, performed statistical analysis, and edited the manuscript; R.A., E.R.S., Y.Z., A.Z., and M.K. performed experiments, collected, and interpreted data; A.H.L. provided CD44 antibodies and interpreted data; H.N. supplied mice; and J.W.S. provided financial resources, designed research, analyzed and interpreted data, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John W. Semple, St. Michael’s Hospital, 30 Bond St, Toronto, ON, Canada M5B 1W8; e-mail: semplej@smh.ca.