Key Points
MINK1 promotes hemostasis and thrombosis in vivo.
MINK1 specifically regulates platelet dense-granule secretion.
Abstract
The sterile-20 kinase misshapen/Nck-interacting kinase (NIK)–related kinase 1 (MINK1) is involved in many important cellular processes such as growth, cytoskeletal rearrangement, and motility. Here, with MINK1-deficient (MINK1−/−) mice, we showed that MINK1 plays an important role in hemostasis and thrombosis via the regulation of platelet functions. In the tail-bleeding assay, MINK1−/− mice exhibited a longer bleeding time than wild-type (WT) mice (575.2 ± 59.7 seconds vs 419.6 ± 66.9 seconds). In a model of ferric chloride–induced mesenteric arteriolar thrombosis, vessel occlusion times were twice as long in MINK1−/− mice as in WT mice. In an in vitro microfluidic whole-blood perfusion assay, thrombus formation on a collagen matrix under arterial shear conditions was significantly reduced in MINK1−/− platelets. Moreover, MINK1−/− platelets demonstrated impaired aggregation and secretion in response to low doses of thrombin and collagen. Furthermore, platelet spreading on fibrinogen was largely hampered in MINK1−/− platelets. The functional differences of MINK1−/− platelets could be attributed to impaired adenosine 5′-diphosphate secretion. Signaling events associated with MINK1 appeared to involve extracellular signal-regulated kinase, p38, and Akt. Hence, MINK1 may be an important signaling molecule that mediates mitogen-activated protein kinase signaling and participates in platelet activation and thrombus formation.
Introduction
Platelets are essential for thrombosis and hemostasis. When vascular integrity is disrupted, platelets instantly activate, undergo adhesion and aggregation, and, finally, form a hemostatic or thrombotic plug. The activation of platelets is initiated by an interplay between platelet receptors and exposed subendothelial matrix proteins; key events in this process include glycoprotein Ib-IX-V (GPIb-IX-V) binding to von Willebrand factor and collagen activating its receptors GPVI and α2β1. Platelets are further recruited and activated by the amplification loops mediated via the effects of subsequently generated or released soluble platelet agonists, mainly adenosine 5′-diphosphate (ADP), thrombin, and thromboxane A2 (TXA2).1 The initial interactions and the secondary stimuli evoke coordinated inside-out intracellular signaling events, leading to a conformational change of integrin αIIbβ3 and subsequent binding to its ligands, von Willebrand factor and fibrinogen. The engagement of these ligands induces outside-in signaling of αIIbβ3, which not only mediates firm platelet adhesion and platelet-platelet aggregation, but also stabilizes the thrombi. Both inside-out and outside-in signaling typically involve several crucial pathways, such as protein kinase C, phosphatidylinositol 3-kinase (PI3K), and mitogen-activated protein kinases (MAPKs).2
Platelets are equipped with 3 major subgroups of MAPKs: extracellular signal-regulated kinase 1/2 (ERK1/2), p38, and c-Jun NH2-terminal kinases (JNK1, 2, 3).3 Recently, platelet MAPKs have become a focus of research on their roles in dominating diverse platelet functions, including adhesion,4 aggregation,5,6 TXA2 synthesis,5,7-9 granule secretion,5,6 and clot retraction.6 Moreover, nearly all platelet stimuli work by activating 1 or more MAPKs. These findings suggest that platelet MAPKs are subject to regulation by a complex network. Conventional theory has established the MAPK activation cascade as a sequential activated triple-kinase module: a MAPK kinase kinase (MAP3K), a MAPK kinase (MAP2K), and a MAPK.10 However, the triple-kinase module is largely based on signaling studies carried out in nucleated cell types; much less is known about how MAPKs are specifically and selectively regulated in anucleate platelets. Furthermore, the functional redundancy and crosstalk between the different MAPK subgroups in platelets indicate the existence of “lurking” players in the hierarchy that selectively organize MAPKs.
Misshapen/Nck-interacting kinase–related kinase 1 (MINK1) is a putatively expressed serine-threonine protein kinase that belongs to the germinal center kinase IV family.11 Previous studies have shown that MINK1 plays crucial roles in fundamental cellular processes such as spreading, cell-matrix adhesion, migration, and cytokinesis.11,12 Interestingly, MINK1 has been reported to be essential in the activation of p38 and JNK, 2 pivotal MAPKs in mediating oncogene Ras-induced ovarian epithelial cell growth arrest13 and the negative selection of autoreactive thymocytes.14 Although previous studies indicate that MINK1 may work as a MAPK kinase kinase kinase in the activation of MAPKs, the arrangement of the signaling cascade does not seem to conform to a unified scenario, but rather is subject to a tissue-specific regulation. For example, in ovarian epithelial cells, MINK1 works downstream of Ras/Raf/ERK and upstream of p3813 ; whereas in thymocytes, it is located downstream of Nck and upstream of JNK.14 Moreover, depending on the tissue type, MINK1 may affect MAPKs via indirect pathways such as transforming growth factor-β/Smad15 or Wnt pathways.16 Given the importance of MAPKs in platelet function and the role of MINK1 as an upstream regulator of MAPKs, MINK1 may be a novel player in platelet function and thus participate in hemostasis and thrombosis. However, a specific role for MINK1 in platelet function has not yet been defined.
In this study, with MINK1-deficient (MINK1−/−) mice, we demonstrate that MINK1 plays an important role in hemostasis and thrombosis via the function of platelets. MINK1−/− platelets displayed impaired dense-granule secretion, which in turn was translated into defective aggregation in response to low doses of thrombin and collagen, and spreading on fibrinogen. Additionally, signaling events associated with MINK1 appeared to involve MAPKs (p38 and ERK1/2) and Akt.
Methods
Generation of MINK1-deficient mice
The MINK1 gene is located on mouse chr11. It has 31 exons and spans a 50-kb genome sequence. The second, third, and fourth exons encode the kinase domain and have an exon-intron structure suitable to make a deletion. As shown in supplemental Figure 1 (see supplemental Data, available on the Blood Web site), a Neor gene in the loxP-frt cassette was inserted into the XhoI site between exons 4 and 5. Another loxP site was put into the SmaI site before exon 3; 129 embryonic stem (ES) cell TC1 was then electroporated with the linearized target DNA construct and the recombinant allele was screened by polymerase chain reaction and Southern blot analysis. Selected ES cell clones were used for microinjection into blastocysts. The resultant chimeras were genotyped and crossed to C57BL/6 mice and the offspring were crossed to a transgenic mouse line carrying ACTFLPe to delete the neomycin resistance. The cre-encoding transgene under the control of adenovirus EIIa-Cre was crossed to generate MINK1−/− mice.
Hematologic analysis
Complete blood counts and hematocrits were determined with an automatic cell counter (Sysmex F-820), using the standard parameters for mice.
Tail-bleeding time
As previously described,5 tails of anesthetized mice were cut 0.5 cm from the tip and immediately immersed in saline (37°C). The time taken for the bleeding to stop (no blood flow for 1 minute) was recorded. Tail-bleeding assays were stopped at 900 seconds if the bleeding did not stop. Data were analyzed by the 2-tailed Mann-Whitney test.
Platelet preparation
Whole blood was collected from the inferior vena cava into 0.1 vol of ACD buffer (75 mM sodium citrate, 39 mM citric acid, and 135 mM dextrose, pH 6.5), and was diluted 1:2 with modified Tyrode buffer (in mM: 20 N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid [HEPES], 137 NaCl, 13.8 NaHCO3, 2.5 KCl, 0.36 NaH2PO4, 5.5 glucose, pH 7.4). Diluted whole blood was centrifuged at 180g for 10 minutes at room temperature, and platelet-rich plasma was collected into a fresh tube. The platelet-rich plasma was diluted in ACD buffer, and centrifuged at 700g for 10 minutes. The platelet pellet was then resuspended in modified Tyrode buffer.
Electron microscopy
Washed wild-type (WT) or MINK1−/− platelets were fixed with 2.5% glutaraldehyde in modified Tyrode buffer. After fixation, staining, and dehydration, the platelets were infiltrated in embedding medium. Thin sections were stained with uranyl acetate and lead citrate. Samples were examined at 80 kV using a Tecnai 10 transmission electron microscope (FEI), and images were captured with an ES500W (782) camera (Gatan) using Digital micrograph software (Gatan).
Flow cytometric analysis
WT or MINK1−/− platelets (106) were labeled for 10 minutes at room temperature with different specific antibodies: fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD41 monoclonal antibody (mAb) (MWReg30; BD Biosciences), FITC-conjugated anti-mouse CD42b mAb (Xia.B2; Emfret Analytics GmbH & Co.KG), FITC-conjugated anti-mouse GPVI mAb (JAQ1; Emfret Analytics). The samples were analyzed with a flow cytometer (EPICS XL; Beckman Coulter).
Platelet aggregation and secretion
Platelet aggregation and secretion were measured using a lumiaggregometer (Chrono-Log) at 37°C with stirring (1200 rpm). Washed platelets (2 × 108/mL) in modified Tyrode buffer were stimulated with thrombin, collagen, U46619, and ADP (in the presence of fibrinogen). Platelet secretion was monitored in parallel with aggregation as adenosine triphosphate (ATP) release with the addition of luciferin/luciferase reagent to the platelet suspension. Inhibitor was incubated with the platelets for 5 minutes prior to stimulation.
Platelet spreading on fibrinogen
Glass coverslips were coated with 20 μg/mL fibrinogen in 0.1 M NaHCO3 (pH 8.3) at 4°C overnight. Washed platelets (2 × 107/mL) or platelets preincubated with 0.1 U/mL apyrase or 1 μM ADP for 5 minutes at 37°C were allowed to spread on the fibrinogen-coated surfaces at 37°C for 60 minutes. After 3 washes with phosphate-buffered saline (PBS), the platelets were fixed, permeabilized, and stained with fluorescein-labeled phalloidin (Molecular Probes), as previously described.17 Adherent platelets were viewed with an inverted fluorescence microscope (Nikon Ti-S) using an S Plan Fluor lens (100×/1.30 numerical aperture oil objective). Images were acquired using a Nikon DS-Qi1-U3 camera. The platelet-covered area was measured using NIS-D software (Nikon).
Platelet-mediated clot retraction
For clot retraction, mouse platelets were processed as previously described.6 Murine platelets were resuspended using citrated human platelet-depleted plasma to a concentration of 4 × 108/mL. Recombined plasma was induced to coagulate by stimulation with thrombin (0.4 U/mL). The clots were allowed to retract at room temperature and photographed at various times.
Immunoblotting
Washed WT or MINK1−/− platelets (250 μL; 2 × 108/mL) were stimulated with collagen and thrombin in a Chrono-Log aggregometer. After 3 minutes of stimulation, the reaction was stopped by adding 2× lysis buffer (50 mM Tris and 150 mM NaCl, pH 7.4) containing 2× protease inhibitor and 2× phosphatase inhibitor. Proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes, which were incubated with primary antibodies. After incubation with the corresponding secondary antibodies (horseradish peroxidase–conjugated immunoglobulin Gs; Jackson ImmunoResearch Laboratories), proteins were visualized by enhanced chemiluminescence and imaged using a Syngene G:BOX Chemi XR system and GeneSnap software (Syngene).
In vitro thrombus formation under flow conditions
Thrombus formation was evaluated in a microfluidic whole-blood perfusion assay on a fibrillar collagen matrix under arterial shear conditions (a shear rate of 1500 s−1) using a Bioflux-200 system (Fluxion). Bioflux plates were coated with fibrillar collagen at 100 μg/mL overnight, and after blocking with 5% bovine serum albumin in PBS for 15 minutes, plates were placed on an inverted microscope and 0.4 mL of mepacrine-labeled blood applied to the inlet well. A shear force of 40 dynes/cm2 was applied, and the platelets were allowed to adhere to collagen for 5 minutes. Adherent platelets were viewed with an inverted fluorescence microscope using an S Plan Fluor lens (20×/0.4 numerical aperture objective). Images were acquired with a Nikon DS-Qi1-U3 CCD camera. The platelet-covered area was measured using Bioflux software (Fluxion).
FeCl3-induced thrombosis
Arterial arterioles were injured with 10% FeCl3, as previously described.17 Anesthetized mice were catheterized via the jugular vein, and fluorescently labeled platelets (108 platelets) were injected through the catheter. The intestines were exposed, and the mesentery was spread on the translucent stage of the fluorescence microscope. Injury of mesenteric arterioles (60-80 μm in diameter) was induced by topical application of 10% FeCl3. Arterioles were monitored for 90 minutes or until complete occlusion occurred (blood flow stopped for >1 minute).
Statistical analysis
Results are expressed as mean ± standard error of the mean (SEM). Statistical significance was evaluated with Student t tests or Mann-Whitney U tests using statistical software (GraphPad Prism; GraphPad Software).
Results
MINK1−/− mice demonstrate impaired hemostasis
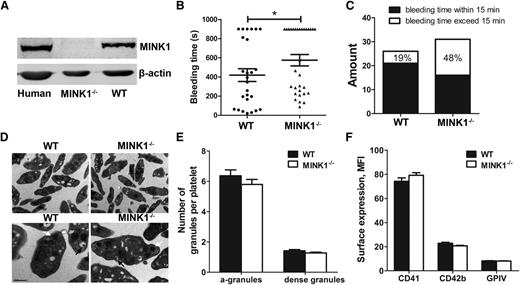
MINK1 was expressed in both human and mouse platelets, and the expression of MINK1 was ablated in MINK1−/− platelets (Figure 1A). MINK1−/− mice were viable and fertile, and did not exhibit any evident bleeding tendency or thrombotic events over their lifespan. MINK1−/− and WT littermates also did not differ significantly in platelet counts, white cell counts, hematocrits, and hemoglobin concentrations (Table 1). The role of MINK1 in hemostasis was investigated in a tail-bleeding assay. MINK1−/− mice exhibited significantly prolonged tail-bleeding times (575.2 ± 59.70 seconds vs 419.6 ± 66.9 seconds; P < .05; Figure 1B). Moreover, 48% of the MINK1−/− mice had bleeding times >15 minutes, compared with 19% of WT littermates (Figure 1C). These data suggested an essential role of MINK1 in hemostasis.
MINK1−/− mice display features of unstable hemostasis in vivo. (A) Analysis of MINK1 expression in platelets from human, WT, and MINK1−/− mice. (B) Bleeding times for WT (●) and MINK1−/− mice (▲). Means are indicated by horizontal lines. Statistical significance was evaluated with 2-tailed Mann-Whitney U tests (*P < .05). (C) Percentages of WT and MINK1−/− mouse bleeding times exceeded 15 minutes (□) or were within 15 minutes (▪). Results were obtained from 26 WT and 31 MINK1−/− mice. (D) Electron microscopic images of WT and MINK1−/− platelet ultrastructure (black arrows, dense granules; white arrows, α-granules). Scale bars, 1 μm (top) and 0.5 μm (bottom). (E) Quantification of α-granules and dense granules of WT (▪) and MINK1−/− (□) platelets. Under ×24 000 magnification, α-granules and dense-granules in 160 platelets (80 from WT and MINK1−/− each) were counted. The data were expressed as mean ± SEM. Statistical significance was evaluated with the Student t test. (F) Surface expression of CD41, CD42b, and GPVI, as determined by flow cytometry with FITC-conjugated anti-mouse CD41 mAb (MWReg30), FITC-conjugated anti-mouse CD42b mAb (Xia.B2), and FITC-conjugated anti-mouse GPVI mAb (JAQ1). Results are expressed as mean fluorescence intensity (MFI), and are the mean ± SEM. Statistical significance was evaluated with the Student t test.
MINK1−/− mice display features of unstable hemostasis in vivo. (A) Analysis of MINK1 expression in platelets from human, WT, and MINK1−/− mice. (B) Bleeding times for WT (●) and MINK1−/− mice (▲). Means are indicated by horizontal lines. Statistical significance was evaluated with 2-tailed Mann-Whitney U tests (*P < .05). (C) Percentages of WT and MINK1−/− mouse bleeding times exceeded 15 minutes (□) or were within 15 minutes (▪). Results were obtained from 26 WT and 31 MINK1−/− mice. (D) Electron microscopic images of WT and MINK1−/− platelet ultrastructure (black arrows, dense granules; white arrows, α-granules). Scale bars, 1 μm (top) and 0.5 μm (bottom). (E) Quantification of α-granules and dense granules of WT (▪) and MINK1−/− (□) platelets. Under ×24 000 magnification, α-granules and dense-granules in 160 platelets (80 from WT and MINK1−/− each) were counted. The data were expressed as mean ± SEM. Statistical significance was evaluated with the Student t test. (F) Surface expression of CD41, CD42b, and GPVI, as determined by flow cytometry with FITC-conjugated anti-mouse CD41 mAb (MWReg30), FITC-conjugated anti-mouse CD42b mAb (Xia.B2), and FITC-conjugated anti-mouse GPVI mAb (JAQ1). Results are expressed as mean fluorescence intensity (MFI), and are the mean ± SEM. Statistical significance was evaluated with the Student t test.
Electron microscopy showed that MINK1−/− platelets had normal discoid morphology. The numbers of α-granules and dense granules were comparable in WT and MINK1−/− platelets (Figure 1D-E). No significant differences in the surface expression of the platelet glycoproteins GPVI, CD41 (αIIb subunit), and CD42b (GPIbα subunit) were found between WT and MINK1−/− platelets (Figure 1F).
MINK1−/− mice display impaired thrombus formation
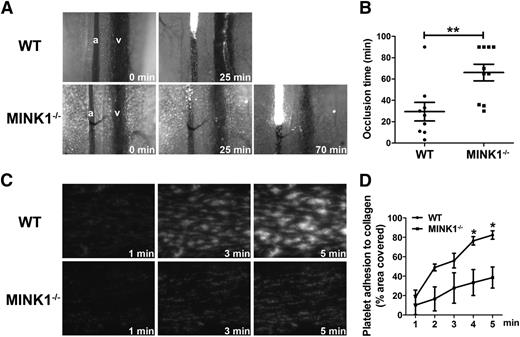
Thrombus formation in vivo was investigated by a FeCl3-induced mesenteric arteriole thrombosis model. The time to formation of a stable occlusive thrombus in a mesenteric arteriole was significantly longer in MINK1−/− mice than in WT mice (65.75 ± 9.88 minutes vs 29.44 ± 8.70 minutes, P < .05; Figure 2A-B), indicating that MINK1 is essential for thrombus formation in vivo.
MINK1−/− mice display impaired thrombus formation in vitro and in vivo. (A) Representative images and time courses of thrombus formation induced by FeCl3 injury to mesenteric arterioles in WT (top row) and MINK1−/− mice (bottom row). a, arteriole; v, venule. (B) Dot plot showing occlusion times for arterioles as a result of FeCl3-induced thrombosis in WT (n = 9) and MINK1−/− mice (n = 10). Means are indicated by horizontal lines. **P < .01, 2-tailed Mann-Whitney test. (C) Photomicrographs showing the progression of adhesion of platelets from WT and MINK1−/− mice on collagen. Whole blood from WT and MINK1−/− mice, collected in heparin (7.5 U/mL), was fluorescently labeled by incubation with mepacrine (100 μM) for 30 minutes, and then perfused through fibrillar collagen-coated bioflux plates at a shear rate of 40 dynes/cm2 for 5 minutes. Original magnification, ×20. (D) Dot plot showing area coverage of platelets from WT and MINK1−/− mice (n = 3 for each group; *P < .05, Student t test).
MINK1−/− mice display impaired thrombus formation in vitro and in vivo. (A) Representative images and time courses of thrombus formation induced by FeCl3 injury to mesenteric arterioles in WT (top row) and MINK1−/− mice (bottom row). a, arteriole; v, venule. (B) Dot plot showing occlusion times for arterioles as a result of FeCl3-induced thrombosis in WT (n = 9) and MINK1−/− mice (n = 10). Means are indicated by horizontal lines. **P < .01, 2-tailed Mann-Whitney test. (C) Photomicrographs showing the progression of adhesion of platelets from WT and MINK1−/− mice on collagen. Whole blood from WT and MINK1−/− mice, collected in heparin (7.5 U/mL), was fluorescently labeled by incubation with mepacrine (100 μM) for 30 minutes, and then perfused through fibrillar collagen-coated bioflux plates at a shear rate of 40 dynes/cm2 for 5 minutes. Original magnification, ×20. (D) Dot plot showing area coverage of platelets from WT and MINK1−/− mice (n = 3 for each group; *P < .05, Student t test).
We next assessed the role of platelet MINK1 in thrombus formation under arterial flow conditions using an in vitro microfluidic whole-blood perfusion assay. Throughout the perfusion time (5 minutes), thrombi formed by MINK1−/− platelets were significantly smaller than those by WT platelets (Figure 2C-D). A recombinant whole-blood system with washed platelets (2 × 107/mL) was also used in the perfusion assay. Under such conditions, platelet aggregation was prevented, which allowed the assessment of platelet adhesion to collagen matrix. MINK1−/− platelets did not demonstrate a reduced adhesion to collagen, compared with WT platelets (supplemental Figure 2). These findings indicated that MINK1 is important in regulating the growth of platelet thrombi under flow conditions.
MINK1−/− platelets display reduced aggregation and impaired dense-granule secretion
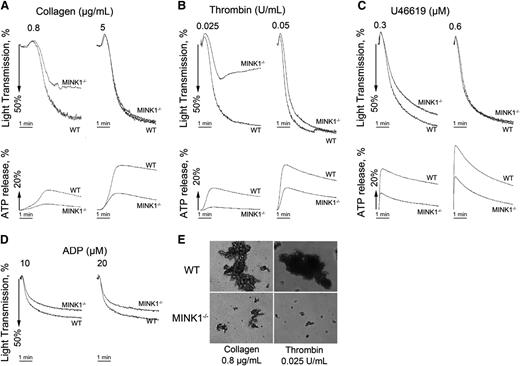
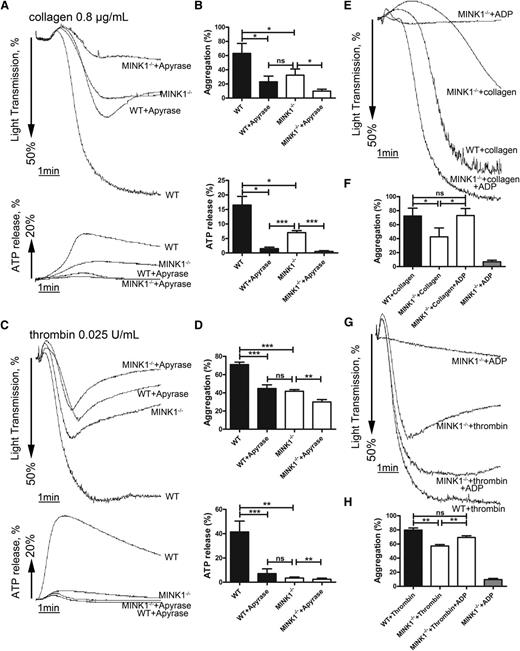
To further characterize the role of MINK1 in the formation of platelet thrombi, platelet aggregation in response to common platelet stimuli was analyzed. Thrombin (0.025 U/mL), collagen (0.8 μg/mL), and the TXA2 analog U46619 (0.3 μM) induced 54%, 49%, and 13% lower aggregation rates in MINK1−/− platelets than in WT platelets (Figure 3A-C; supplemental Figure 3A-C). Under these conditions, dense-granule secretion was largely inhibited in MINK1−/− platelets (Figure 3A-C; supplemental Figure 3A-C). Higher concentrations of thrombin (0.05 U/mL), collagen (5 μg/mL), and U46619 (0.6 μM) overcame the defective aggregation, but not the defective dense-granule secretion in MINK1−/− platelets (Figure 3A-C; supplemental Figure 3A-C), despite normal total ADP and serotonin content in MINK1−/− platelets (supplemental Figure 4). ADP-induced platelet aggregation was not affected by MINK1 deficiency (Figure 3D; supplemental Figure 3D). The secretion of α-granules, measured by P-selectin expression, was not influenced by MINK1 deficiency (supplemental Figure 5A). By measuring the binding of JON/A antibody, MINK1 deficiency did not affect the activation of αIIbβ3 on single platelets (supplemental Figure 5B). Moreover, collagen and thrombin-induced TXA2 synthesis were normal in MINK1−/− platelets (supplemental Figure 6). Collectively, these data indicate that MINK1 does not affect the initial inside-out signaling elicited by agonists, but rather controls platelet dense-granule secretion and thus plays roles in the amplification phase of thrombus formation. Consistently, MINK1−/− platelets stimulated with low doses of thrombin and collagen only formed microaggregates (Figure 3E). Furthermore, the aggregation of WT platelets induced by thrombin and collagen was reduced to a level comparable to MINK1−/− platelets when ADP was hydrolyzed by apyrase (Figure 4A-D). We then determined whether supplementing ADP could rescue the defective aggregation in MINK1−/− platelets. Indeed, supplementation with a low concentration of granule-content ADP (1 μM), insufficient to induce aggregation on its own, reversed the inhibitory effect of MINK1 deficiency on collagen- and thrombin-stimulated platelet aggregation (Figure 4E-H). These data further confirm that the impaired aggregation in MINK1−/− platelets is caused by the reduced ADP secretion.
MINK1−/− platelets show impaired aggregation responses associated with defective release of ATP. (A-D) Platelets were stimulated with collagen, thrombin, the TXA2 analog U46619, and ADP (in the presence of fibrinogen). Aggregation and ATP release was assessed with a Chrono-log lumiaggregometer under stirring at 1200 rpm for 5 minutes. Traces are representative of at least 3 independent experiments. (E) Platelets were then fixed with 1.5% paraformaldehyde for 30 minutes at room temperature, and observed by differential interference contrast (Nikon TE-2000S, ×10 objective, DS-2MBWc-U1 CCD camera). Data are representative of 3 independent experiments.
MINK1−/− platelets show impaired aggregation responses associated with defective release of ATP. (A-D) Platelets were stimulated with collagen, thrombin, the TXA2 analog U46619, and ADP (in the presence of fibrinogen). Aggregation and ATP release was assessed with a Chrono-log lumiaggregometer under stirring at 1200 rpm for 5 minutes. Traces are representative of at least 3 independent experiments. (E) Platelets were then fixed with 1.5% paraformaldehyde for 30 minutes at room temperature, and observed by differential interference contrast (Nikon TE-2000S, ×10 objective, DS-2MBWc-U1 CCD camera). Data are representative of 3 independent experiments.
Reduced ADP secretion is responsible for defective aggregation of MINK1−/− platelets. (A,C) Aggregation and ATP release of washed WT or MINK1−/− platelets stimulated with collagen (0.8 μg/mL) or thrombin (0.025 U/mL) in the presence or absence of apyrase (1 U/mL) incubated for 5 minutes. (B,D) Percentage of platelet aggregation and ATP release from at least 4 independent experiments depicted in panels A and C, and the results are shown as mean ± SEM (*P < .05, **P < .01, ***P < .001, paired Student t test). (E,G) A low concentration of ADP (1 μM), insufficient to induce aggregation on its own, reversed the inhibitory effect of MINK1−/− platelets on aggregation stimulated with collagen (0.8 μg/mL) or thrombin (0.025 U/mL). (F,H) Percentage of platelet aggregation from at least 4 independent experiments depicted in panels E and G, and the results are shown as mean ± SEM (*P < .05, **P < .01, paired Student t test). ns, no significance.
Reduced ADP secretion is responsible for defective aggregation of MINK1−/− platelets. (A,C) Aggregation and ATP release of washed WT or MINK1−/− platelets stimulated with collagen (0.8 μg/mL) or thrombin (0.025 U/mL) in the presence or absence of apyrase (1 U/mL) incubated for 5 minutes. (B,D) Percentage of platelet aggregation and ATP release from at least 4 independent experiments depicted in panels A and C, and the results are shown as mean ± SEM (*P < .05, **P < .01, ***P < .001, paired Student t test). (E,G) A low concentration of ADP (1 μM), insufficient to induce aggregation on its own, reversed the inhibitory effect of MINK1−/− platelets on aggregation stimulated with collagen (0.8 μg/mL) or thrombin (0.025 U/mL). (F,H) Percentage of platelet aggregation from at least 4 independent experiments depicted in panels E and G, and the results are shown as mean ± SEM (*P < .05, **P < .01, paired Student t test). ns, no significance.
MINK1−/− platelets exhibit reduced spreading on immobilized fibrinogen
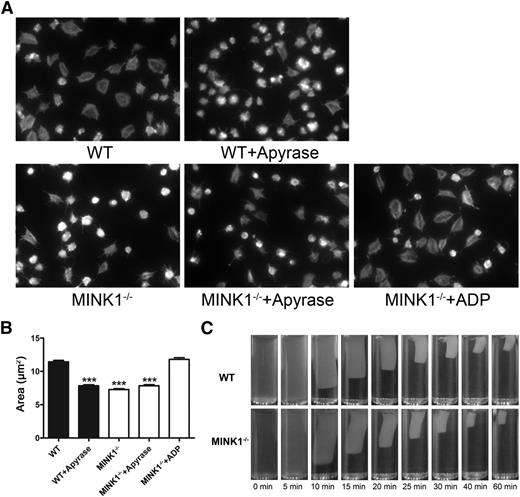
Platelet spreading on immobilized fibrinogen and clot retraction, 2 processes controlled by early and late integrin αIIbβ3-mediated outside-in signaling, respectively,18-20 were then measured. Spreading on fibrinogen was largely hampered by MINK1 deficiency (Figure 5A-B). The defective spreading was also caused by impaired ADP secretion, as apyrase eliminated the difference in spreading between WT and MINK1−/− platelets, and a subthreshold dose of ADP rescued the impaired spreading caused by MINK1 deficiency (Figure 5A-B). However, clot retraction mediated by WT and MINK1−/− platelets did not show any difference (Figure 5C). Taken together, these data suggested that MINK1 contributes to early outside-in signaling by promoting ADP secretion.
MINK1 regulates platelet spreading, but not clot retraction. (A) Spreading of WT and MINK1−/− platelets on immobilized fibrinogen in the presence or absence of apyrase (1 U/mL) or ADP (1 μM). Images are representative of 3 independent experiments with similar results. Original magnification, ×100. (B) Quantification of the areas of spread (μm2) WT and MINK1−/− platelets (mean ± SEM; ***P < .001, Student t test). (C) Platelets from WT or MINK1−/− mice were resuspended with human platelet-poor plasma at a concentration of 4 × 108/mL, and recombined plasma was stimulated to coagulate with thrombin (0.4 U/mL), then photographed at different time points. Experiments were repeated at least 3 times.
MINK1 regulates platelet spreading, but not clot retraction. (A) Spreading of WT and MINK1−/− platelets on immobilized fibrinogen in the presence or absence of apyrase (1 U/mL) or ADP (1 μM). Images are representative of 3 independent experiments with similar results. Original magnification, ×100. (B) Quantification of the areas of spread (μm2) WT and MINK1−/− platelets (mean ± SEM; ***P < .001, Student t test). (C) Platelets from WT or MINK1−/− mice were resuspended with human platelet-poor plasma at a concentration of 4 × 108/mL, and recombined plasma was stimulated to coagulate with thrombin (0.4 U/mL), then photographed at different time points. Experiments were repeated at least 3 times.
MINK1 regulates the activation of ERK, p38, and Akt in platelets stimulated by collagen and thrombin
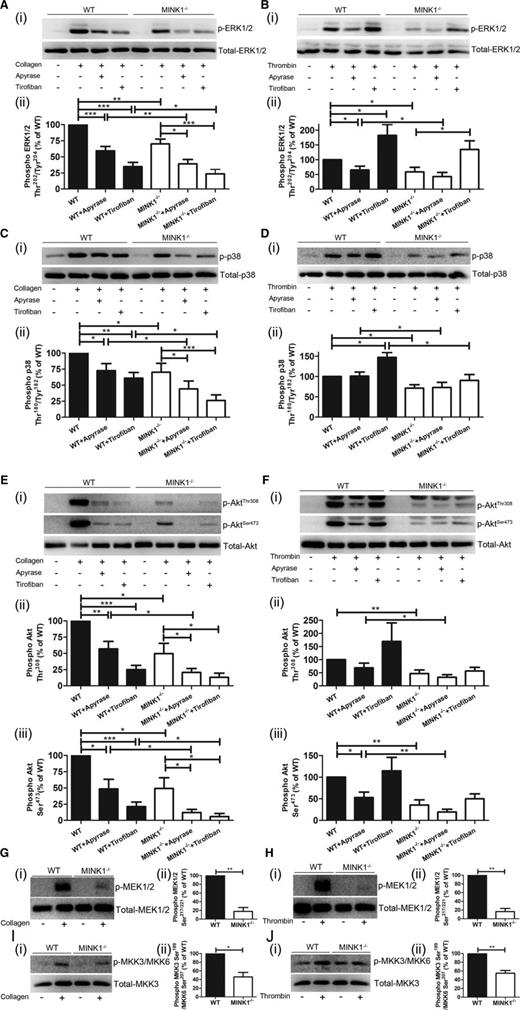
When cellular signaling in platelets mediated by MINK1 was analyzed, phosphorylation of ERK (Thr202/Tyr204) and p38 (Thr180/Tyr182) induced by collagen and thrombin was significantly reduced in MINK1−/− platelets (Figure 6A-D). However, JNK phosphorylation was unaffected (supplemental Figure 7). The phosphorylation of MAPK/ERK kinase (MEK)1/2 (Ser183/221) and MAPK kinase 3 (MKK3) (Ser189)/MKK6 (Ser207), MAP2Ks upstream of ERK1/2 and p38,21 was also partially reduced by MINK1 deficiency upon collagen and thrombin stimulation (Figure 6G-J). However, phosphorylation of MAP3Ks (c-Raf, mix-lineage kinase 3, MAPK/ERK kinase kinase 1 [MEKK1], and apoptosis signal-regulating kinase 1), previously reported upstream of MEK1/2 and MKK3/6,21,22 did not differ between WT and MINK1−/− platelets (supplemental Figure 8). Nevertheless, limited by the availability of antibodies, we could not exclude the possibility that other MAP3Ks such as MEKK3 and dual-leucine-zipper-bearing kinase 122 may participate as downstream effectors of MINK1. Moreover, phosphorylation of Akt (Thr308 and Ser473) was also largely decreased in MINK1−/− platelets in response to collagen and thrombin (Figure 6E-F). Notably, although the phosphorylation levels of ERK, p38, and Akt were largely diminished by apyrase, the remaining phosphorylation levels of these molecules were still significantly lower in MINK1−/− platelets (Figure 6A-F). And the decreased phosphorylation of ERK, p38, and Akt was fully recovered by exogenous ADP (supplemental Figure 9). This indicated that MINK1 regulates the phosphorylation of ERK, p38, and Akt, independent of secreted ADP.
MINK1 deficiency impairs ERK, p38, Akt, MEK1/2, and MKK3/MKK6 phosphorylation. (Ai,Ci,Ei,Gi,Ii) Immunoblot analysis of WT and MINK1−/− platelets, stimulated with collagen (0.8 μg/mL) for 3 minutes in the absence or presence of apyrase (1 U/mL) or tirofiban (2 μg/mL), with antibodies recognizing phosphorylated ERK1/2 Thr202/Tyr204 (T202/Y204), total ERK1/2, phosphorylated p38 Thr180/Tyr182 (T180/Y182), total p38, phosphorylated Akt Thr308, phosphorylated Akt Ser473, total Akt, phosphorylated MEK1/2 Ser183/221, total MEK1/2, phosphorylated MKK3 Ser189/MKK6 Ser207, and total MKK3. (Aii-iii,Cii-iii,Eii-iii,Gii-iii,Iii-iii) Results were quantified and presented as mean ± SEM from at least 3 independent experiments (*P < .05, **P < .01, ***P < .001; paired Student t test). (Bi,Di,Fi,Hi,Ji) Immunoblot analysis of WT and MINK1−/− platelets, stimulated with thrombin (0.025 U/mL) for 3 minutes in the absence or presence of apyrase (1 U/mL) or tirofiban (2 μg/mL), with antibodies recognizing phosphorylated ERK1/2 Thr202/Tyr204 (T202/Y204), total ERK1/2, phosphorylated p38 Thr180/Tyr182 (T180/Y182), total p38, phosphorylated Akt Thr308, phosphorylated Akt Ser473, total Akt, phosphorylated MEK1/2 Ser183/221, total MEK1/2, phosphorylated MKK3 Ser189/MKK6 Ser207, and total MKK3. (Bii-iii,Dii-iii,Fii-iii,Hii-iii,Jii-iii) Results were quantified and presented as mean ± SEM from at least 3 independent experiments (*P < .05, **P < .01; paired Student t test).
MINK1 deficiency impairs ERK, p38, Akt, MEK1/2, and MKK3/MKK6 phosphorylation. (Ai,Ci,Ei,Gi,Ii) Immunoblot analysis of WT and MINK1−/− platelets, stimulated with collagen (0.8 μg/mL) for 3 minutes in the absence or presence of apyrase (1 U/mL) or tirofiban (2 μg/mL), with antibodies recognizing phosphorylated ERK1/2 Thr202/Tyr204 (T202/Y204), total ERK1/2, phosphorylated p38 Thr180/Tyr182 (T180/Y182), total p38, phosphorylated Akt Thr308, phosphorylated Akt Ser473, total Akt, phosphorylated MEK1/2 Ser183/221, total MEK1/2, phosphorylated MKK3 Ser189/MKK6 Ser207, and total MKK3. (Aii-iii,Cii-iii,Eii-iii,Gii-iii,Iii-iii) Results were quantified and presented as mean ± SEM from at least 3 independent experiments (*P < .05, **P < .01, ***P < .001; paired Student t test). (Bi,Di,Fi,Hi,Ji) Immunoblot analysis of WT and MINK1−/− platelets, stimulated with thrombin (0.025 U/mL) for 3 minutes in the absence or presence of apyrase (1 U/mL) or tirofiban (2 μg/mL), with antibodies recognizing phosphorylated ERK1/2 Thr202/Tyr204 (T202/Y204), total ERK1/2, phosphorylated p38 Thr180/Tyr182 (T180/Y182), total p38, phosphorylated Akt Thr308, phosphorylated Akt Ser473, total Akt, phosphorylated MEK1/2 Ser183/221, total MEK1/2, phosphorylated MKK3 Ser189/MKK6 Ser207, and total MKK3. (Bii-iii,Dii-iii,Fii-iii,Hii-iii,Jii-iii) Results were quantified and presented as mean ± SEM from at least 3 independent experiments (*P < .05, **P < .01; paired Student t test).
MINK1-regulated phosphorylation of ERK, p38, and Akt in response to collagen and thrombin was also measured in the presence of the integrin αIIbβ3 inhibitor tirofiban. In both WT and MINK1−/− platelets, tirofiban greatly reduced the collagen-stimulated, while enhancing the thrombin-stimulated, phosphorylation of ERK, p38, and Akt (Figure 6A-F). However, the phosphorylation levels of these molecules were significantly lower in MINK1−/− platelets than in WT platelets. These results suggested that the MINK1-regulated phosphorylation of ERK, p38, and Akt is independent of integrin αIIbβ3-mediated outside-in signaling.
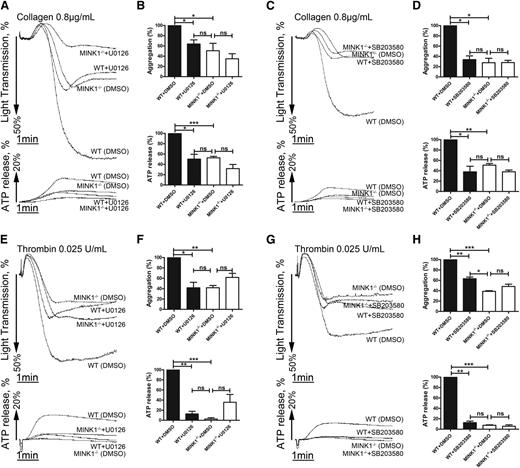
Decreased activation of ERK and p38 is responsible for the impaired aggregation of MINK1−/− platelets
Finally, we examined the effects of ERK and p38 on MINK1-regulated platelet activation. The MEK1/2 inhibitor U0126 and the p38 inhibitor SB203580 inhibited the collagen- and thrombin-induced aggregation of WT platelets, but not of MINK1−/− platelets (Figure 7). Moreover, collagen- and thrombin-stimulated dense-granule secretion was reduced by U0126 and SB203580 in WT platelets, but not in MINK1−/− platelets. Therefore, MINK1 is functionally coupled to ERK and p38 activation.
Defective ERK and p38 phosphorylation are responsible for impaired platelet aggregation of MINK1−/− platelets. (A,C,E,G) Aggregation and ATP release of WT or MINK1−/− platelets stimulated with collagen (0.8 μg/mL) or thrombin (0.025 U/mL) incubated in the presence of dimethylsulfoxide (DMSO), U0126 (10 μM), or SB203580 (10 μM) for 5 minutes. (B,D,F,H) The bar chart shows means ± SEM of the percentage of platelet aggregation and ATP release from at least 3 independent experiments depicted in panels A, C, E, and G (*P < .05, **P < .01 between WT and MINK1−/− mice, paired Student t test).
Defective ERK and p38 phosphorylation are responsible for impaired platelet aggregation of MINK1−/− platelets. (A,C,E,G) Aggregation and ATP release of WT or MINK1−/− platelets stimulated with collagen (0.8 μg/mL) or thrombin (0.025 U/mL) incubated in the presence of dimethylsulfoxide (DMSO), U0126 (10 μM), or SB203580 (10 μM) for 5 minutes. (B,D,F,H) The bar chart shows means ± SEM of the percentage of platelet aggregation and ATP release from at least 3 independent experiments depicted in panels A, C, E, and G (*P < .05, **P < .01 between WT and MINK1−/− mice, paired Student t test).
Discussion
In the present study, we investigated the role of MINK1 in platelet function and thrombosis. MINK1 deficiency led to impaired hemostasis and thrombus formation in vivo. In vitro, MINK1−/− platelets displayed a phenotype of defective aggregation, dense-granule secretion, and spreading on immobilized fibrinogen. Interestingly, defects in dense-granule secretion seem to be the primary reason for the dysfunction of MINK1−/− platelets. Moreover, MINK1 appears to target ERK, p38, and Akt phosphorylation to regulate platelet activation.
Our study, for the first time, revealed that MINK1 is an important molecule in the hemostatic and thrombotic systems. MINK1 deficiency caused prolonged tail-bleeding time and occlusion time in arterial thrombosis, despite the normal hematologic parameters. Although it is arguable that possible vasculature defects upon MINK1 deficiency might contribute, the phenotype is most likely explained by compromised platelet function. Clear evidence lies in the results from the whole-blood perfusion assay, which showed that MINK1−/− platelets fail to form large thrombi over a collagen surface under arterial flow condition. The analysis of isolated platelets further substantiated a direct effect of MINK1 on platelet functions.
Our data revealed a highly specific role for MINK1 in controlling dense-granule secretion in response to both G-protein–coupled receptor agonists (thrombin, U46619) and an immunoreceptor tyrosine-based activation motif receptor agonist (collagen). This was evidenced by the following observations in this study. First, MINK1 deficiency did not affect the inside-out activation of integrin αIIbβ3 and P-selectin expression in stimulated single platelets. Second, MINK1 deficiency did not influence platelet aggregation stimulated by ADP, a well-known agonist unable to induce dense-granule secretion,23 whereas dense-granule secretion induced by thrombin, U46619, and collagen was clearly hampered in MINK1−/− platelets. Third, only the secretion-dependent second wave of aggregation, but not the first wave induced by collagen and thrombin, was affected by MINK1 deficiency. And the defective aggregation was reversed by supplementation with a low concentration of dense-granule-content ADP. It is noteworthy that although apyrase further inhibited aggregation of MINK1−/− platelets, it failed to further influence MINK1−/− platelets to spread on fibrinogen. This seeming discrepancy may be explained by the differences of cell density and cell-cell interaction mode between aggregation and spreading conditions. Under aggregation conditions, despite reduced ADP secretion by MINK1 deletion, the high density of platelets and tight interaction still enabled the accumulation of a sufficiently high concentration of extracellular ADP, whose effects were demonstrable in the presence of apyrase. However, under spreading conditions, because of much lower platelet density and the absence of tight cellular interaction, the concentration of remaining secreted ADP upon MINK1 deletion became too negligible to sustain an observable spreading. The absence of ADP in the spreading conditions was further supported by the fact that the impaired spreading of MINK1−/− platelets on immobilized fibrinogen was fully corrected by low concentration of ADP. Thus, MINK1 appears to be essential in promoting dense-granule secretion.
Despite the importance of dense-granule secretion in platelet function,24 signaling pathways specifically regulating this process are not well defined. Although a definitive involvement of MAP3Ks remains to be elucidated, the present study showed that MINK1 acts upstream of MEK1/2, MKK3/6, p38, ERK, and Akt, sequentially and specifically regulating dense-granule secretion. These results are consistent with reports that the MAPK pathway5,25,26 and the PI3K/Akt pathway27,28 stimulate platelet secretion. Interestingly, MINK1 is not involved in the regulation of integrin αIIbβ3 per se, as evidenced by (1) normal JON/A binding on stimulated MINK1−/− platelets and (2) unaltered tyrosine phosphorylation of the β3 cytoplasmic domain (Tyr759 and Tyr747) and Src-family kinases (Tyr416) in MINK1−/− platelets upon Mn2+ stimulation and spreading on immobilized fibrinogen (supplemental Figure 10). Moreover, the MINK1-controlled signaling pathway does not seem to depend on integrin αIIbβ3 outside-in signaling, as tirofiban failed to eliminate the phosphorylation differences of ERK, p38, and Akt between WT and MINK1−/− platelets stimulated with collagen and thrombin. It is intriguing that MINK1 deficiency did not influence α-granule secretion, although it has been reported that MAPKs are important in both α-granule secretion and dense-granule secretion.6 One possible explanation might be that the pool of an activated MAPK is further divided and directed to the functional regulation of these 2 types of granules through recruiting specific further downstream signaling molecules. Indeed, previous studies have provided evidence that secretion of these 2 types of granules in platelets is differentially regulated. For example, integrin-linked kinase has been reported to support α-granule secretion while sparing the secretion of dense granules29 ; disruption of actin polymerization has been shown to preferentially affect α-granule secretion.30 Moreover, as shown in the present study, MINK1 deficiency reduced but did not abolish the phosphorylation of ERK, p38, and Akt, indicating that only a proportion of these molecules are subject to MINK1 control and participate in dense-granule secretion, whereas the remaining MINK1-unrelated proportions of activated MAPKs may be associated with other platelet activation processes, such as α-granule secretion6 and integrin αIIbβ3 activation.31,32 Future work is clearly needed to pinpoint MINK1-regulated molecules further downstream of MAPKs.
To summarize, we have identified platelet MINK1 as a novel player in hemostasis and thrombosis. Via the regulation of MAPKs and PI3K/Akt pathways, MINK1 plays a highly specific role in controlling platelet dense-granule secretion. Future work on this interesting molecule may help to unlock the mystery of platelet dense-granule secretion.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Mingzhu Zheng for assistance in mouse strain maintenance, Dr Beibei Wang for technical assistance with electron microscopy, and Prof Lain Bruce for linguistic advice.
This work was supported by grants from the National Natural Science Foundation of China (81370618 and 81170478) and the National Basic Research Program of China (2012CB966603).
Authorship
Contribution: M.Y., D.L., and S.Y. designed and performed experiments and analyzed data; M.Y. wrote the manuscript; P.L. conducted flow cytometry; M.H. helped to generate the MINK1-knockout mice; Y.L. helped with western blotting; Q.Z., S.W., Q.H., and Y.N. supplied reagents and revised the manuscript; and H.H. and L.L. designed the research and wrote and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hu Hu, Laboratory for Thrombosis and Haemostasis, Department of Pathology and Pathophysiology, Zhejiang University School of Medicine, Hangzhou 310058, China; e-mail: huhu@zju.edu.cn; or Linrong Lu, Institute of Immunology, Zhejiang University School of Medicine, Hangzhou 310058, China; e-mail: lu_linrong@zju.edu.cn.
References
Author notes
M.Y. and D.L. contributed equally to the work.