Key Points
Anti-EBV TCR-like monoclonal antibodies reduce BLCLs tumor load in vivo.
Anti-EBV TCR-like monoclonal antibodies mediate phagocytosis of BLCLs by macrophages.
Abstract
Epstein-Barr virus (EBV) is an oncovirus associated with several human malignancies including posttransplant lymphoproliferative disease in immunosuppressed patients. We show here that anti-EBV T-cell receptor–like monoclonal antibodies (TCR-like mAbs) E1, L1, and L2 bound to their respective HLA-A*0201-restricted EBV peptides EBNA1562-570, LMP1125-133, and LMP2A426-434 with high affinities and specificities. These mAbs recognized endogenously presented targets on EBV B lymphoblastoid cell lines (BLCLs), but not peripheral blood mononuclear cells, from which they were derived. Furthermore, these mAbs displayed similar binding activities on several BLCLs, despite inherent heterogeneity between different donor samples. A single weekly administration of the naked mAbs reduced splenomegaly, liver tumor spots, and tumor burden in BLCL-engrafted immunodeficient NOD-SCID/Il2rg−/− mice. In particular, mice that were treated with the E1 mAb displayed a delayed weight loss and significantly prolonged survival. In vitro, these TCR-like mAbs induced early apoptosis of BLCLs, thereby enhancing their Fc-dependent phagocytic uptake by macrophages. These data provide evidence for TCR-like mAbs as potential therapeutic modalities to target EBV-associated diseases.
Introduction
Epstein-Barr virus (EBV) is a human gammaherpesvirus found in more than 90% of the human population. Apart from being the etiological agent of infectious mononucleosis, EBV is also associated with a number of human malignancies such as Burkitt’s lymphoma, Hodgkin’s lymphoma, nasopharyngeal carcinoma, and posttransplant lymphoproliferative disorder (PTLD) in immunosuppressed transplant recipients. EBV infects B cells in vivo and persists as a lifelong, asymptomatic latent infection in immunocompetent individuals with functional T-cell immunosurveillance. However, in transplant recipients, the administration of immunosuppressants to prevent graft rejection perturbs the balance between T cells and EBV-infected B cells. As a result, the unrestricted proliferation of these EBV-infected cells can lead to EBV-PTLD, which is characterized by the expression of all 9 EBV latent proteins (also known as the latency III program).1
PTLD is a life-threatening disease with high mortality rates. Although various treatment modalities are available for EBV-PTLD, there is a lack of consensus on a standard treatment regime. Rituximab (anti-CD20) is the antibody most commonly used to treat PTLD, and its usage is regarded as one of the most successful approaches.2-4 Yet rituximab can inadvertently deplete noninfected healthy B cells, as the expression of CD20 is not exclusive to EBV-infected malignant B cells. Treatment with rituximab has also been associated with an increased risk for opportunistic infections.5,6 Furthermore, rituximab can potentially drive the development of CD20− lymphoma in patients undergoing therapy, thereby rendering the treatment ineffective.7,8 The clinical efficacy of rituximab has nevertheless demonstrated the feasibility of antibody-based treatment of PTLD and has highlighted the need for antibodies with greater EBV-targeting specificities and reduced adverse effects.
T-cell receptor–like monoclonal antibodies (TCR-like mAbs) exquisitely recognize a specific peptide presented on a major histocompatibility complex (MHC) molecule, akin to a TCR. TCR-like mAbs not only possess the fine specificity of T-cell–like recognition but also enable the targeting of intracellular, often hidden viral or tumor antigens based on their surface display as peptide epitopes. As antibodies, TCR-like mAbs also exhibit better stabilities and higher affinities than TCRs.9 Over the years, increasing interest in TCR-like mAbs has led to a rapid expansion of the repertoire of TCR-like mAbs targeting both viral10-13 and tumor-associated antigens.14-19 These studies have demonstrated the feasibility of TCR-like mAbs to target cells expressing neoantigen, independent of the immunoregulatory microenvironment that might inhibit T-cell function.14 These studies validate the usage of TCR-like mAbs as therapeutic agents that can specifically target surface antigens, in association with MHC, which are highly expressed by cancer cells.
We have previously reported the generation of 3 novel TCR-like mAbs against EBV latent proteins. These antibodies were shown to bind to their respective targets EBNA1562–570 (FMVFLQTHI) (E1), LMP1125–133 (YLLEMLWRL) (L1), and LMP2A426–434 (CLGGLLTMV) (L2) in association with human leukocyte antigen (HLA)-A*0201 with high affinities and specificities. These TCR-like mAbs were shown to detect endogenous targets found on EBV-positive cell lines, splenic lesions of EBV-infected humanized mice, as well as nasopharyngeal carcinoma biopsies, underscoring their ability to recognize these EBV epitopes in EBV-associated malignancies.13
Despite the ability to recognize endogenous EBV antigens, the therapeutic potential of these antibodies in targeting EBV-associated tumors has not been directly assessed. B lymphoblastoid cell lines (BLCLs) are the in vitro counterparts of B cells found in EBV-PTLD, with both displaying the characteristic latency III expression pattern. Furthermore, the intravenous engraftment of BLCLs into immunocompromised mice is a basic model of EBV-PTLD that is often used for therapeutic testing, with injected BLCLs infiltrating organs commonly affected in PTLD, including liver and spleen.20-23
Here, we show that TCR-like mAbs E1, L1, and L2 bind endogenous targets on various HLA-A*0201+ donor EBV-infected BLCLs, but not the peripheral blood mononuclear cells (PBMCs) from which they were derived. In vivo, a weekly single administration of E1, L1, and L2 resulted in a reduction of BLCLs in engrafted immunodeficient mice. Importantly, engrafted mice that received the E1 TCR-like mAb displayed a delayed weight loss and improved survival. Mechanistically, TCR-like mAbs, particularly E1, mediated an upregulation of phosphatidylserine on BLCLs, leading to enhanced phagocytic uptake by macrophages. These results thus demonstrate the potential usage of TCR-like mAbs in the targeting of EBV-associated malignancies.
Materials and methods
pMHC ELISA
Recombinant biotinylated human HLA-A*0201 carrying UV cleavable peptides were subjected to UV in the presence of the peptides of interest to facilitate exchange, as previously described.24 Biotinylated peptide–MHC (pMHC) complexes were then added into streptavidin-coated enzyme-linked immunosorbent assay (ELISA) wells in quadruplicates. TCR-like mAbs were serially diluted and incubated with the pMHC complexes before detection with horseradish peroxidase-conjugated goat anti-mouse secondary antibody (ThermoScientific). 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) substrate solution (Invitrogen) was added, and reactions were stopped with 1% sodium azide and 0.1 M citric acid. Absorbance was measured at 415 nm on a spectrophotometer, and graphs (nonlinear regression) were plotted on GraphPad Prism software.
BLCLs generation
Blood samples were obtained with informed consent from HLA-typed healthy donors. The National University of Singapore Institutional Review Board approved of this study (DSRB/E/2008/00293). PBMCs were isolated by Ficoll Paque (GE Healthcare) and infected with EBV B95-8 supernatant, washed, and subsequently cultured in RPMI-1640 containing 10% fetal bovine serum, 1% penicillin/streptomycin supplemented with 1 μg/mL cyclosporin A (Sigma). Cyclosporin A was withdrawn after 2 weeks and BLCLs were established with further culturing.25,26
Flow cytometry
5 × 105 cells were resuspended in flow cytometry buffer (phosphate-buffered saline supplemented with 3% fetal bovine serum 0.01% sodium azide) and incubated with TCR-like mAbs. Cells were then washed and incubated with AlexaFluor-488 goat anti-mouse IgG (H + L) antibodies (Life Technologies). For PBMCs analysis, cells were further stained with PE-Cy7 anti-CD19 (HIB19), APC-Cy7 anti-CD3 (HIT3a), APC anti-CD56 (HCD56), and PE anti-HLA-A2 (BB7.2) (BioLegend). For the xenograft model, single cells were obtained by homogenizing organs through a 70-μm cell strainer. Cell pellets were treated with ammonium-chloride-potassium lysing buffer (Gibco) and were washed twice with phosphate-buffered saline before staining with fluorescein isothiocyanate anti-human CD45 (HI30) and PE anti-mouse CD45.1 (A20) antibodies in flow cytometry buffer. All samples were acquired on BD LSRFortessa (BD Biosciences) and analyzed using FlowJo software (TreeStar). For immunophenotyping of BLCLs, refer to supplemental Methods, available on the Blood Web site.
Xenograft model
Twelve- to 15-week-old NOD-SCID/Il2rg−/− (NSG) mice were intravenously injected with 2 × 106 BLCLs via the tail vein. One week later, mice were administered the TCR-like mAbs (300 μg/mouse) or immunoglobulin G1 (IgG1) isotype control antibody (BioLegend) once a week for up to 5 weeks. Mice were monitored for changes in body weight and killed when values fell below 20% of their initial weight. Animal experiments were conducted under the provisions of the National University of Singapore Institutional Animal Care and Use Committee (protocol 122/11).
Histology
Formalin-fixed, paraffin-embedded tissue organs were sectioned to 4 μm thickness for staining with hematoxylin and eosin. Images were captured using a Carl Zeiss MIRAX MIDI slide scanner. Analysis was done using a Pannoramic Viewer (3DHISTECH).
Phagocytosis assay
Bone marrow–derived macrophages were adhered onto coverslips before assay (macrophage preparation detailed in supplemental Methods). BLCLs, if stated, were incubated for 24 hours at 37°C with 100 μg/mL of the respective antibodies in Opti-MEM media before assay. Thereafter, BLCLs were labeled with carboxyfluorescein succinimidyl ester (CFSE) and incubated with the respective antibodies at 10 μg/mL for 3 hours at 4°C before co-culturing with macrophages for 4 hours at 37°C. Cells were harvested and stained with anti-mouse CD11b-PE (BD Pharmingen) for flow cytometry. Phagocytosed cells were defined as CFSE+CD11b+ population. For imaging, cells were fixed in 4% paraformaldehyde and blocked with phosphate-buffered saline containing 3% fetal bovine serum, 0.5% mouse serum. Cells were then stained with anti-mouse CD11b-PE and Hoechst dye and mounted onto glass slides with ProLong Gold Antifade reagent. Images were captured using Olympus IX81 inverted microscope with Metamorph software at 40× objective. At least 200 macrophages were counted per sample. Phagocytic index was calculated based on (% macrophages with at least 1 BLCL) × (average number of BLCLs per phagocytic cell).
Annexin-V assay
105 cells were cultured in Opti-MEM media and were treated with 10 μg/mL or 100 μg/mL TCR-like mAbs, mouse IgG1 isotype control, or 1 μM camptothecin as positive control. Cells were harvested after 6 or 24 hours and washed and stained with annexin-V-Pacific Blue Conjugate (Life Technologies) before acquisition via flow cytometry.
Site-directed mutagenesis of D265A antibodies
Anti-EBV TCR-like mAbs (FcγR null binding variant) with D265A point mutation were generated by replacing aspartate at position 265 with alanine, using the QuikChange Site-Directed Mutagenesis Kit (Stratagene), in accordance with the manufacturer’s instructions.
Statistical analysis
Statistical analysis was performed using GraphPad Prism software, applying Student t test. Log-rank test was used for Kaplan-Meier survival analysis.
Results
TCR-like mAbs E1, L1, and L2 are specific for their pMHC targets
We previously described the generation of 3 TCR-like mAbs targeting EBV latent epitopes presented on HLA-A*0201 through modifications to the conventional hybridoma technology.13 Briefly, splenocytes from immunized mice were magnetically enriched with the specific biotinylated pMHC complexes before fusion, resulting in an enhanced percentage of hybridomas secreting target-specific antibodies.13
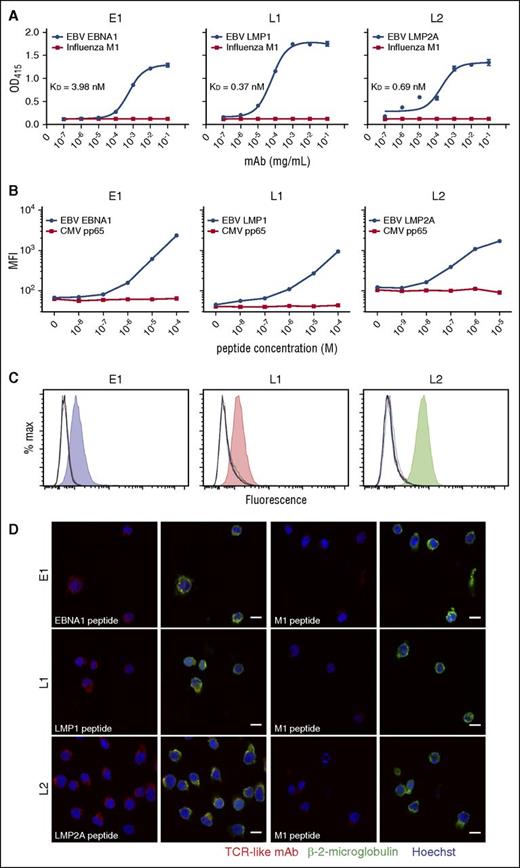
To demonstrate the specificities of these TCR-like mAbs for their pMHC, we used a pMHC ELISA with HLA-A*0201 monomers substituted with the relevant peptides.24 TCR-like mAbs E1, L1, and L2 displayed antibody concentration-dependent binding for their respective epitopes EBNA1562-570 (dissociation constant [KD] = 3.98 nM), LMP1125-133 (KD = 0.37 nM), and LMP2A426-434 (KD = 0.69 nM), but not for the control M158-66 peptide (Figure 1A). L1 displayed the best binding avidity (lowest KD value) among the 3 antibodies, consistent with our earlier SPR data.13 These results indicate that our TCR-like mAbs bind specifically to their respective pMHC complexes, akin to that of a TCR.
Binding specificities of TCR-like mAbs E1, L1, and L2 as determined by peptide-MHC ELISA, flow cytometry, and confocal microscopy. (A) Biotinylated MHC complexes containing UV-cleavable surrogate peptides were exchanged with the respective peptides and coated onto streptavidin-containing ELISA wells. TCR-like mAbs were diluted 10-fold and incubated with the peptide-MHC complexes before detection and readout at OD 415 nm. (B) Peptides were diluted 10-fold and pulsed onto T2 cells before staining with TCR-like mAbs and fluorescein isothiocyanate-conjugated goat anti-mouse secondary antibodies for detection via flow cytometry. (C) C1R-A2 cells were acid-stripped and pulsed with 10 different peptides EBV EBNA1562-570 (blue), LMP1125-133 (red), LMP2A426-434 (green), Influenza M158-66 (orange), cytomegalovirus pp65495-503 (cyan), IE181-89 (magenta), HIV pol476-484 (brown), gp120120-128 (purple), Mycobacterium tuberculosis Ag85B143-152 (dark green), hepatitis B virus sAg183-191 (navy blue), in addition to unpulsed control (black). Shaded histograms represent the relevant peptides pulsed for each TCR-like mAb staining. (D) Confocal microscope images of T2 cells pulsed with relevant (first and second columns) and control M1 peptide (third and fourth columns) before staining and secondary antibodies detection with TCR-like mAbs (R-phycoerythrin–conjugated goat anti-mouse IgG), β-2-microglobulin (AlexaFluor-488-conjugated goat anti-rabbit IgG), and Hoechst dye (blue). Images were acquired using Zeiss LSM510 confocal microscope software version 2.5 SP2 under 63× oil immersion objective at 512 × 512 resolution. Images were overlayed using ImageJ. Scale bars, 10 μm. MFI, mean fluorescence intensity.
Binding specificities of TCR-like mAbs E1, L1, and L2 as determined by peptide-MHC ELISA, flow cytometry, and confocal microscopy. (A) Biotinylated MHC complexes containing UV-cleavable surrogate peptides were exchanged with the respective peptides and coated onto streptavidin-containing ELISA wells. TCR-like mAbs were diluted 10-fold and incubated with the peptide-MHC complexes before detection and readout at OD 415 nm. (B) Peptides were diluted 10-fold and pulsed onto T2 cells before staining with TCR-like mAbs and fluorescein isothiocyanate-conjugated goat anti-mouse secondary antibodies for detection via flow cytometry. (C) C1R-A2 cells were acid-stripped and pulsed with 10 different peptides EBV EBNA1562-570 (blue), LMP1125-133 (red), LMP2A426-434 (green), Influenza M158-66 (orange), cytomegalovirus pp65495-503 (cyan), IE181-89 (magenta), HIV pol476-484 (brown), gp120120-128 (purple), Mycobacterium tuberculosis Ag85B143-152 (dark green), hepatitis B virus sAg183-191 (navy blue), in addition to unpulsed control (black). Shaded histograms represent the relevant peptides pulsed for each TCR-like mAb staining. (D) Confocal microscope images of T2 cells pulsed with relevant (first and second columns) and control M1 peptide (third and fourth columns) before staining and secondary antibodies detection with TCR-like mAbs (R-phycoerythrin–conjugated goat anti-mouse IgG), β-2-microglobulin (AlexaFluor-488-conjugated goat anti-rabbit IgG), and Hoechst dye (blue). Images were acquired using Zeiss LSM510 confocal microscope software version 2.5 SP2 under 63× oil immersion objective at 512 × 512 resolution. Images were overlayed using ImageJ. Scale bars, 10 μm. MFI, mean fluorescence intensity.
We next serially titrated the peptides and pulsed them onto T2 cells. Likewise, the TCR-like mAbs showed peptide concentration-dependent binding to their respective peptides, but not to an irrelevant cytomegalovirus pp65495-503 peptide, with discernible differences even at peptide concentrations in the nanomolar range (Figure 1B). Furthermore, these antibodies displayed excellent binding specificities for their respective epitopes EBNA1562-570, LMP1125-133, and LMP2A426-434 associated with HLA-A*0201, but not for 9 other HLA-A*0201-restricted, pathogen-derived peptides pulsed onto C1R-A2 cells (Figure 1C).
To determine that TCR-like mAbs E1, L1, and L2 indeed recognize target antigens found on the cell surface, we visualized their binding on peptide-pulsed T2 cells. Confocal imaging revealed colocalized staining of E1, L1, and L2 with surface β-2-microglobulin for cells pulsed with the specific peptides, but not on those pulsed with the irrelevant M158-66 peptide (Figure 1D). These data altogether reinforce our earlier findings on the binding specificities and avidities of the TCR-like mAbs E1, L1, and L2.
E1, L1, and L2 bind to latency III-expressing BLCLs, but not PBMCs from EBV-seropositive donors
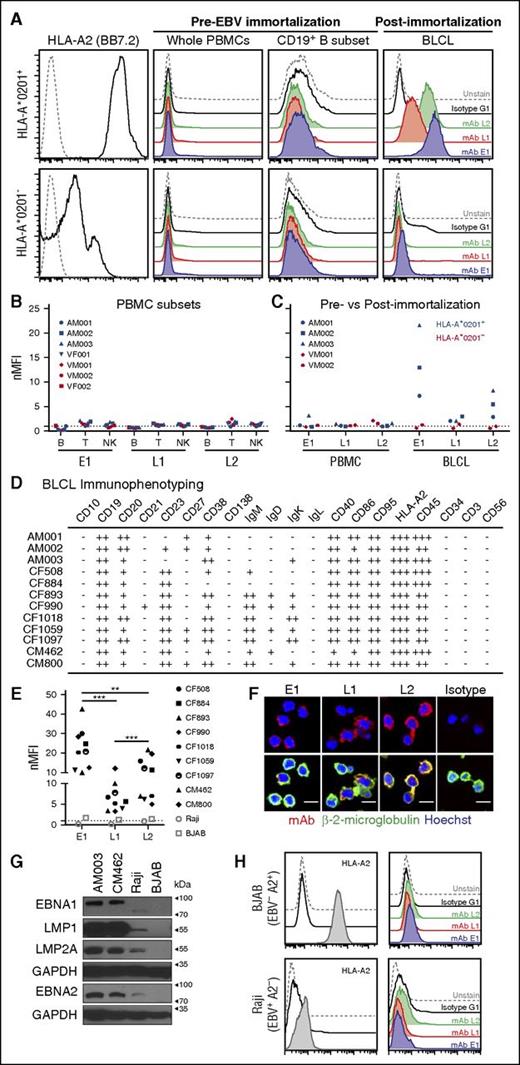
A recurring concern with TCR-like mAbs is their capacity to bind targets only in the presence of saturating exogenous peptides. As such, to determine whether these antibodies could recognize endogenously derived surface pMHC targets, we compared the abilities of these antibodies to detect their respective endogenous antigens on human PBMCs before and after EBV immortalization and establishment of BLCLs. To this end, PBMCs were isolated from HLA-A*0201+ and HLA-A*0201− healthy donors for staining with TCR-like mAbs and markers for different immune subsets. Minimal binding with TCR-like mAbs was observed, regardless of the HLA-A allotypes or within the different immune subsets of PBMCs; namely, B, T, and NK cells that are reportedly known to be potential EBV hosts (Figure 2A-B; supplemental Figure 1). After EBV infection of PBMCs and establishment of BLCLs, we observed an increased binding of the TCR-like mAbs to HLA-A*0201+ EBV BLCLs, but not to its HLA-A*0201− counterpart (Figure 2C). Both BLCLs were indeed of B-cell origin (CD19+CD3−CD56−), as indicated by flow cytometry (supplemental Figure 1B). Notably, E1 displayed the highest staining intensity, followed by L2 and L1 on the HLA-A*0201+ PBMCs-BLCLs pairs tested (Figure 2B-C). These data were consistent with our previous report on the hierarchical dominance of HLA-A2/EBNA1 presentation on EBV-positive cell lines.13
Binding of E1, L1, and L2 to endogenous surface antigens on EBV-transformed BLCLs. (A) Representative histogram staining of HLA-A*0201+ and HLA-A*0201− donor samples with 3 of the TCR-like mAbs: E1 (blue), L1 (red), and L2 (green) before and after EBV infection and establishment of EBV BLCL outgrowth. (B) Normalized mean fluorescence intensity of the CD19+ B, CD3+ T, and CD56+ NK immune cell subsets in PBMCs with the 3 TCR-like mAbs. MFI values were normalized by dividing each MFI reading with their respective isotype controls. (C) nMFI of EBV BLCLs pre- and post-EBV immortalization. Blue and red symbols indicate HLA-A*0201+ and HLA-A*0201− samples, respectively. (D) Immunophenotyping summary of HLA-A*0201+ BLCLs examined in this study. Normalized MFI value of 0 to less than 2 indicates negative staining (−), above 2 to 9.99 (+), 10 to 99.9 (++), and 100 to 1000 (+++). (E) Compilation of normalized mean fluorescence intensity values of each HLA-A*0201+ BLCL from this study, with HLA-A2+ EBV− BJAB (open square) and HLA-A2− EBV+ Raji (open circle) cell lines as controls. Unpaired Student t test was performed to compare the binding profiles between each TCR-like mAb on HLA-A*0201+ BLCLs. **P < .001; ***P < .0001. (F) Confocal microscope images of BLCLs stained with the respective TCR-like mAbs (R-phycoerythrin), β-2-microglobulin (AlexaFluor-488), and Hoechst dye (blue). Scale bars, 10 μm. (G) Immunoblot detection of EBV latent proteins in 2 HLA-A*0201+ BLCLs AM003, CM462, HLA-A*0201− atypical latency III cell line Raji, and EBV− BJAB as a control. GAPDH served as a loading control. (H) Representative histograms staining indicating BJAB as being HLA-A2+ and Raji cell line as HLA-A2−, with TCR-like mAbs staining profiles similar to that of isotype and unstained controls.
Binding of E1, L1, and L2 to endogenous surface antigens on EBV-transformed BLCLs. (A) Representative histogram staining of HLA-A*0201+ and HLA-A*0201− donor samples with 3 of the TCR-like mAbs: E1 (blue), L1 (red), and L2 (green) before and after EBV infection and establishment of EBV BLCL outgrowth. (B) Normalized mean fluorescence intensity of the CD19+ B, CD3+ T, and CD56+ NK immune cell subsets in PBMCs with the 3 TCR-like mAbs. MFI values were normalized by dividing each MFI reading with their respective isotype controls. (C) nMFI of EBV BLCLs pre- and post-EBV immortalization. Blue and red symbols indicate HLA-A*0201+ and HLA-A*0201− samples, respectively. (D) Immunophenotyping summary of HLA-A*0201+ BLCLs examined in this study. Normalized MFI value of 0 to less than 2 indicates negative staining (−), above 2 to 9.99 (+), 10 to 99.9 (++), and 100 to 1000 (+++). (E) Compilation of normalized mean fluorescence intensity values of each HLA-A*0201+ BLCL from this study, with HLA-A2+ EBV− BJAB (open square) and HLA-A2− EBV+ Raji (open circle) cell lines as controls. Unpaired Student t test was performed to compare the binding profiles between each TCR-like mAb on HLA-A*0201+ BLCLs. **P < .001; ***P < .0001. (F) Confocal microscope images of BLCLs stained with the respective TCR-like mAbs (R-phycoerythrin), β-2-microglobulin (AlexaFluor-488), and Hoechst dye (blue). Scale bars, 10 μm. (G) Immunoblot detection of EBV latent proteins in 2 HLA-A*0201+ BLCLs AM003, CM462, HLA-A*0201− atypical latency III cell line Raji, and EBV− BJAB as a control. GAPDH served as a loading control. (H) Representative histograms staining indicating BJAB as being HLA-A2+ and Raji cell line as HLA-A2−, with TCR-like mAbs staining profiles similar to that of isotype and unstained controls.
E1, L1, and L2 bind targets on EBV BLCLs derived from various donors
EBV BLCLs are known to be heterogeneous between individuals. Hence, we next determined whether our TCR-like mAbs could bind to their targets on BLCLs derived from different HLA-A*0201+ donors. Indeed, an immunophenotypic characterization of our existing panel of HLA-A*0201+ BLCLs showed differing B-cell marker expression, highlighting the heterogeneity of EBV-infected B cells (Figure 2D; supplemental Figure 2). Despite such differences, we observed similar binding profiles of our antibodies E1, L1, and L2 across these HLA-A*0201+ BLCLs, with E1 displaying significantly greater staining on BLCLs than the other 2 antibodies (Figure 2E; supplemental Figure 3). These TCR-like mAbs also displayed colocalization with β-2-microglobulin staining on EBV-transformed BLCLs (Figure 2F). Binding of these TCR-like mAbs was EBV and HLA-A*0201-specific, as minimal staining was observed on HLA-A2+ EBV− BJAB and the non-HLA-A2 EBV+ Raji cell line (which also expresses EBNA1, EBNA2, LMP1, and LMP2A) (Figure 2G-H).
TCR-like mAbs treatment reduces tumor load in BLCL-xenograft mouse model
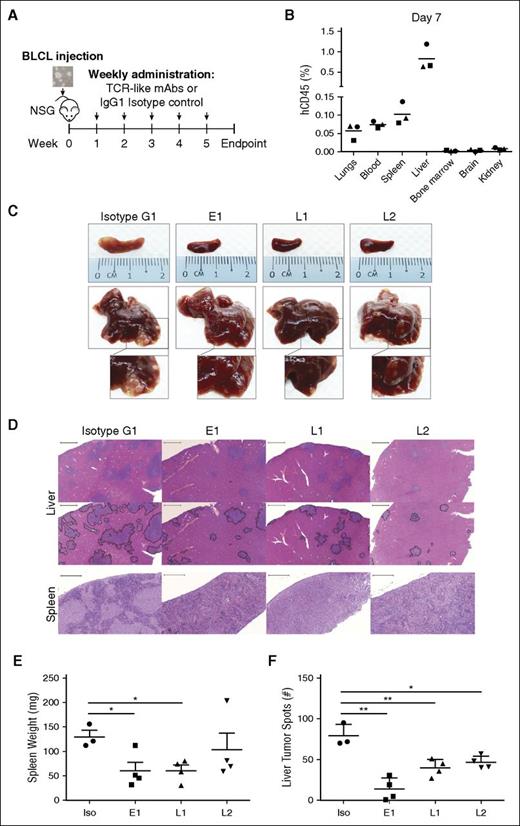
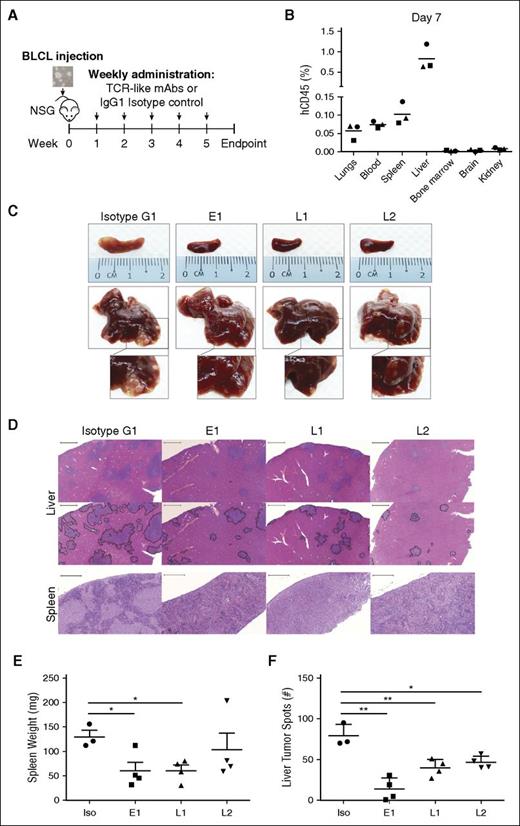
We next tested the ability of our TCR-like mAbs to target EBV BLCLs in vivo (Figure 3A). To simulate PTLD in immunosuppressed transplant recipients, we intravenously engrafted BLCLs into immunodeficient NSG mice and checked for their presence 1 week after engraftment. Although BLCL transplanted mice displayed comparable gross anatomy to their noninjected counterparts, human CD45+ BLCLs were already detectable by flow cytometry in the liver and spleen, indicating successful engraftment (Figure 3B; supplemental Figure 4). With this xenograft model, we tested the antibodies’ therapeutic efficacies through weekly administration for up to 5 weeks post-BLCLs transplantation (Figure 3A).
TCR-like mAbs treatment decreases tumor load in vivo. (A) Schematic illustration of TCR-like mAbs treatment. Two million BLCLs were injected intravenously into NSG mice at week 0 before weekly administration of mAbs (300 μg/mouse) from week 1 onward. (B) Human CD45 (hCD45)-positive BLCLs were detectable in various organs day 7 after injection of BLCLs, indicating BLCL engraftment. (C, D) Mice were killed upon 20% weight loss, and organs were harvested for terminal analysis. Images are representative of gross anatomy and hematoxylin and eosin staining of spleens and livers from mice of each treatment group. Images were taken using Carl Zeiss MIRAX MIDI slide scanner, and analyses were performed using a 3DHISTECH Pannoramic Viewer. Liver tumor spots were traced in black (bottom), using Adobe Photoshop CS6. Scale bars, 500 μm (spleen) and 2000 μm (liver). (E) Spleens were weighed, and (F) the number of liver tumor spots were counted during terminal analysis. Results were expressed as mean ± SD. *P < .05, **P < .01 (unpaired Student t test). Data are representative of 2 independent experiments.
TCR-like mAbs treatment decreases tumor load in vivo. (A) Schematic illustration of TCR-like mAbs treatment. Two million BLCLs were injected intravenously into NSG mice at week 0 before weekly administration of mAbs (300 μg/mouse) from week 1 onward. (B) Human CD45 (hCD45)-positive BLCLs were detectable in various organs day 7 after injection of BLCLs, indicating BLCL engraftment. (C, D) Mice were killed upon 20% weight loss, and organs were harvested for terminal analysis. Images are representative of gross anatomy and hematoxylin and eosin staining of spleens and livers from mice of each treatment group. Images were taken using Carl Zeiss MIRAX MIDI slide scanner, and analyses were performed using a 3DHISTECH Pannoramic Viewer. Liver tumor spots were traced in black (bottom), using Adobe Photoshop CS6. Scale bars, 500 μm (spleen) and 2000 μm (liver). (E) Spleens were weighed, and (F) the number of liver tumor spots were counted during terminal analysis. Results were expressed as mean ± SD. *P < .05, **P < .01 (unpaired Student t test). Data are representative of 2 independent experiments.
As shown in Figure 3C, mice that were treated with the TCR-like mAbs displayed marked reduction in tumor growth in livers and spleens compared with isotype-treated controls. Hematoxylin and eosin staining revealed a reduction in tumor spots with TCR-like mAbs treatment in the livers and a similar decrease of BLCL infiltration within the spleens (Figure 3D). Consistent with the above observations, mice that were treated with the TCR-like mAbs E1 and L1 displayed significantly reduced splenomegaly, and all TCR-like mAbs-treated mice showed significant reductions in total liver tumor spot counts, in comparison with isotype controls (Figure 3E-F).
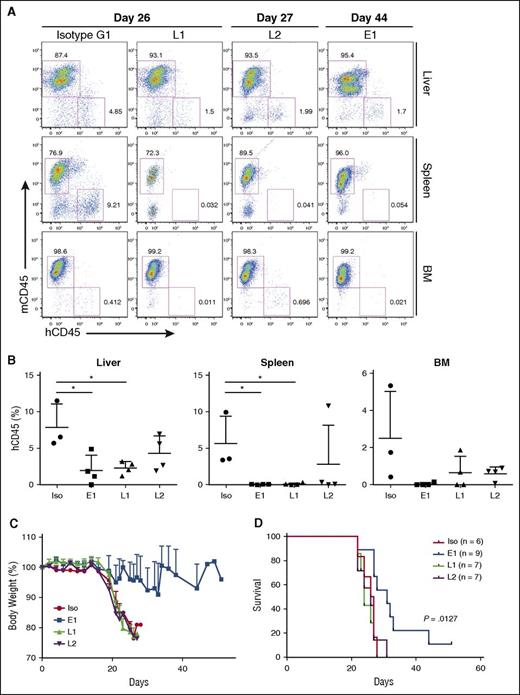
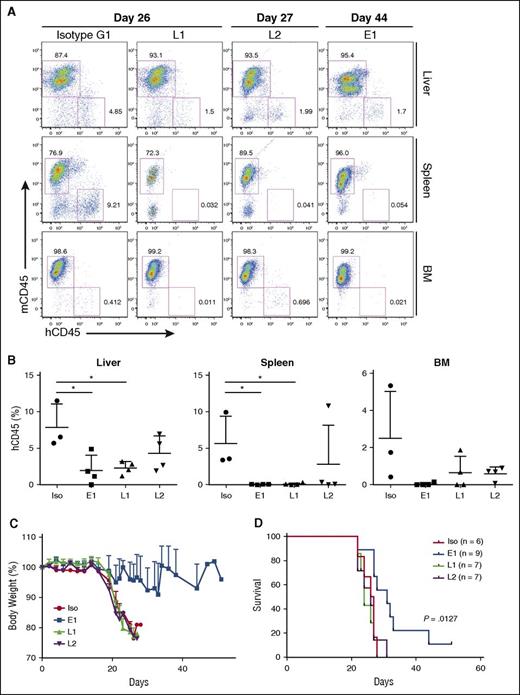
To assess the BLCL-depleting effects of the TCR-like mAbs, organs were harvested and processed for anti-human vs anti-mouse CD45 staining. In agreement with the gross anatomy data, a reduction in percentages of human CD45+ cells was detected in livers (E1: 1.977 ± 2.08%; L1: 2.31 ± 0.89%; L2: 4.3 ± 2.39%; isotype: 7.91 ± 3.14%), spleens (E1: 0.048 ± 0.035%; L1: 0.108 ± 0.137%; L2: 2.819 ± 5.322%: isotype: 5.637 ± 3.737%), and bone marrows (E1: 0.054 ± 0.084%; L1: 0.651 ± 0.88%; L2: 0.606 ± 0.361%; isotype: 2.504 ± 2.535%) of the TCR-like mAbs-treated mice (Figure 4A-B). These data indicated that a single administration of the TCR-like mAbs per week could lead to a reduction of BLCL infiltration in vivo.
TCR-like mAbs treatment reduces BLCL numbers, with E1 conferring delayed weight loss and enhanced survival. (A) Representative hCD45 vs mouse CD45 (mCD45) dot plots for each treatment group, with days post-EBV BLCL engraftment indicated. (B) Compiled percentages of hCD45 cells detected in the livers, spleens, and bone marrow (BM) from terminal analysis of EBV BLCL engrafted NSG mice. Results were expressed as mean ± SD. *P < .05 (unpaired Student t test). (C) Mice were monitored for changes in weight and were killed when values fell beneath 20% of original weight. Data are representative of 2 independent experiments. (D) Kaplan-Meier analysis comparing survival of mice between each treatment group. P value represents log-rank Mantel-Cox test result.
TCR-like mAbs treatment reduces BLCL numbers, with E1 conferring delayed weight loss and enhanced survival. (A) Representative hCD45 vs mouse CD45 (mCD45) dot plots for each treatment group, with days post-EBV BLCL engraftment indicated. (B) Compiled percentages of hCD45 cells detected in the livers, spleens, and bone marrow (BM) from terminal analysis of EBV BLCL engrafted NSG mice. Results were expressed as mean ± SD. *P < .05 (unpaired Student t test). (C) Mice were monitored for changes in weight and were killed when values fell beneath 20% of original weight. Data are representative of 2 independent experiments. (D) Kaplan-Meier analysis comparing survival of mice between each treatment group. P value represents log-rank Mantel-Cox test result.
E1-treated mice display delayed weight loss and improved survival
BLCL-transplanted NSG mice were also monitored for changes in body weight and survival. As shown in Figure 4C-D, although L1 and L2 treatments reduced BLCL infiltration within the assessed organs, they did not translate into improved survival. In contrast, mice that were treated with the E1 TCR-like mAb showed delayed weight loss and significantly improved survival (P = .0127), as shown by Kaplan-Meier analysis. This could be attributed to the comparatively enhanced tumor clearance, as seen earlier with the E1 TCR-like mAb (Figures 3E-F and 4A-B).
TCR-like mAbs exert antitumor activity via triggering early apoptosis and phagocytosis
Therapeutic antibodies have been proposed to mediate tumor cell depletion through several mechanisms, including antibody-dependent cellular cytotoxicity, complement-dependent cytotoxicity, antibody-dependent phagocytosis, and direct triggering of apoptosis.14-16,18,27 As NSG mice lack T, B, and NK cells, but retain innate immune cells, we speculated that the mode of action of our antibodies could involve direct apoptosis and/or phagocytosis.
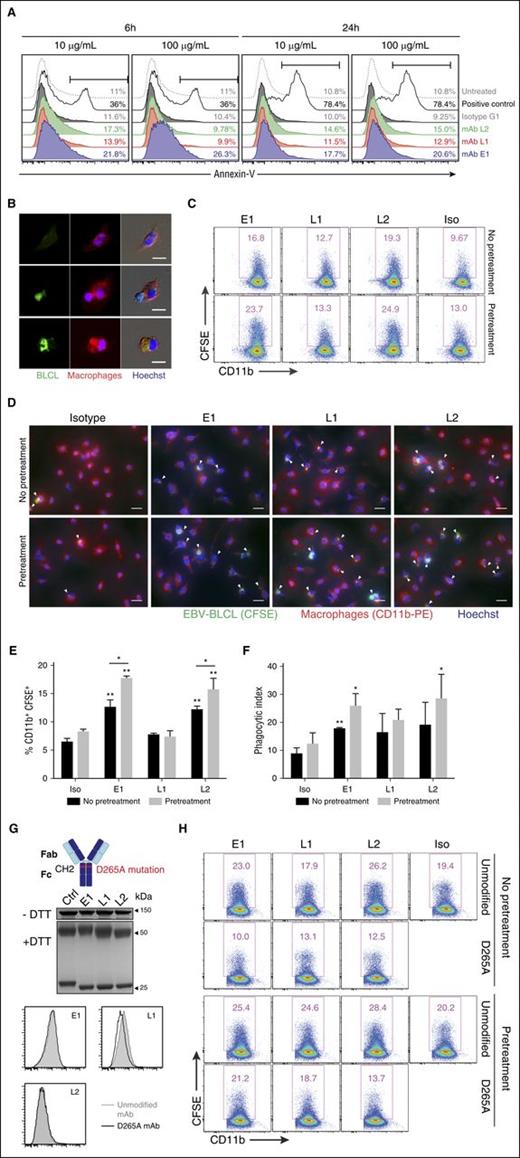
Exposed phosphatidylserine on the cell surface is an indicator of early apoptosis that results from the loss of phospholipid membrane asymmetry. As such, to determine whether E1, L1, and L2 could directly induce apoptosis, BLCLs were cultured with the respective mAbs and stained with annexin-V to assess surface expression of phosphatidylserine.28 Incubation of BLCLs with E1 resulted in an increased detection of phosphatidylserine at both 6 and 24 hours after treatment, in comparison with isotype and untreated controls. Upregulation of phosphatidylserine was also detected, albeit to a lesser extent, for L1 and L2 (Figure 5A). Taken together, these data suggest that the TCR-like mAbs could induce early apoptosis when bound to BLCLs.
In vitro mechanisms of TCR-like mAbs-mediated antitumor activity. (A) Representative annexin-V staining histograms of BLCLs treated with TCR-like mAbs. BLCLs were incubated with 10 or 100 μg/mL mAbs and were harvested at 6 or 24 hours for detection of surface phosphatidylserine via annexin-V staining. Camptothecin (1 μM) was used as a positive control. (B) Immunofluorescence microscopy showing an EBV BLCL (green) in contact with a macrophage (red) (middle), as well as BLCL engulfment by a macrophage (bottom). Images were obtained using Olympus IX81 inverted microscope MetaMorph software at 40× objective. Scale bars, 10 μm. (C) Representative dot plots from phagocytosis assay. BLCLs were preincubated with the mAbs for 24 hours (pretreatment) or without pretreatment before CFSE labeling, further TCR-like mAbs staining, and subsequent coculturing with macrophages. Phagocytosed cells were defined as the CD11b-PE+ CFSE+ population. (D) Immunofluorescence microscopy images visualizing the presence of CFSE-labeled BLCLs within macrophages (as indicated by white arrows) under the stated conditions. Scale bars, 20 μm. (E, F) Tabulated percentages of CD11b+ CFSE+ cells and phagocytic index from phagocytosis assays. Results represent the average of 2 independent experiments, expressed as mean ± SD. *P < .05; **P < .01 (unpaired Student t test). (G, top) Illustration denoting the point mutation from aspartate to alanine at position 265 of the antibody Fc region, abrogating Fc receptor binding. (Middle) Representative SDS-PAGE gels of D265A antibody variants treated with nonreducing (−dithiothreitol) or reducing (+dithiothreitol) conditions. (Bottom) Histogram plots comparing the unmodified mAb and D265A antibody variants of the 3 TCR-like mAbs. (H) Representative flow cytometry plots from phagocytosis assays using the respective unmodified and D265A mAbs.
In vitro mechanisms of TCR-like mAbs-mediated antitumor activity. (A) Representative annexin-V staining histograms of BLCLs treated with TCR-like mAbs. BLCLs were incubated with 10 or 100 μg/mL mAbs and were harvested at 6 or 24 hours for detection of surface phosphatidylserine via annexin-V staining. Camptothecin (1 μM) was used as a positive control. (B) Immunofluorescence microscopy showing an EBV BLCL (green) in contact with a macrophage (red) (middle), as well as BLCL engulfment by a macrophage (bottom). Images were obtained using Olympus IX81 inverted microscope MetaMorph software at 40× objective. Scale bars, 10 μm. (C) Representative dot plots from phagocytosis assay. BLCLs were preincubated with the mAbs for 24 hours (pretreatment) or without pretreatment before CFSE labeling, further TCR-like mAbs staining, and subsequent coculturing with macrophages. Phagocytosed cells were defined as the CD11b-PE+ CFSE+ population. (D) Immunofluorescence microscopy images visualizing the presence of CFSE-labeled BLCLs within macrophages (as indicated by white arrows) under the stated conditions. Scale bars, 20 μm. (E, F) Tabulated percentages of CD11b+ CFSE+ cells and phagocytic index from phagocytosis assays. Results represent the average of 2 independent experiments, expressed as mean ± SD. *P < .05; **P < .01 (unpaired Student t test). (G, top) Illustration denoting the point mutation from aspartate to alanine at position 265 of the antibody Fc region, abrogating Fc receptor binding. (Middle) Representative SDS-PAGE gels of D265A antibody variants treated with nonreducing (−dithiothreitol) or reducing (+dithiothreitol) conditions. (Bottom) Histogram plots comparing the unmodified mAb and D265A antibody variants of the 3 TCR-like mAbs. (H) Representative flow cytometry plots from phagocytosis assays using the respective unmodified and D265A mAbs.
Macrophages are important innate immune cells that can exhibit Fc-dependent antitumor activity via antibody-mediated phagocytosis.29 To check whether the TCR-like mAbs could facilitate such phagocytic uptake, we incubated CFSE-labeled BLCLs with the antibodies and cocultured the cells with macrophages. Immunofluorescence microscopy revealed the presence of CFSE signal within CD11b+ macrophages, indicating the process of BLCL–macrophage contact and engulfment (Figure 5B). This was further confirmed via flow cytometry (Figure 5C,E; no pretreatment panels) and by tabulating phagocytic index (Figure 5D,F; no pretreatment panels), where macrophages displayed improved phagocytic uptake with E1 and L2 treatment in comparison with isotype control.
Upregulation of phosphatidylserine with TCR-like mAb treatment enhances phagocytosis of BLCLs by macrophages
Because cell surface phosphatidylserine on apoptotic targets can elicit their phagocytic uptake, we next tested whether the induction of cell surface phosphatidylserine via pretreatment of TCR-like mAbs could increase the phagocytosis of BLCLs by macrophages.30,31 To this end, BLCLs were preincubated with the respective mAbs for 24 hours to allow the upregulation of phosphatidylserine expression before the phagocytosis assay. Indeed, phagocytosis was augmented for BLCLs that were pretreated with the TCR-like mAbs, particularly for E1 and L2, in comparison with conditions without such prior treatment (Figure 5C-F). A similar but nonsignificant trend was also observed for the tabulated phagocytic index (Figure 5D,F).
TCR-like mAbs-mediated phagocytosis of BLCLs is Fc-dependent
To determine whether TCR-like mAbs-mediated phagocytosis of BLCLs was Fc-dependent, we generated variants containing the Fc null mutation D265A that impairs Fc receptor binding.32-34 As shown in Figure 5G, the introduction of Fc null mutation D265A does not affect their binding capacity when compared with the unmodified TCR-like mAbs. However, phagocytosis was abolished to levels at or below that of the isotype control with the Fc null mAb variants, regardless of whether the BLCLs were pretreated with the mAbs before phagocytosis assay (Figure 5H). These data indicated that Fc receptor binding was required for the phagocytic uptake of BLCLs by macrophages.
Discussion
Here we characterized 3 TCR-like mAbs in targeting EBV epitopes presented on HLA-A*0201+ BLCLs. These TCR-like mAbs demonstrated high binding affinity with KD value in the nanomolar range, superior to that of a TCR binding to its cognate pMHC (0.1-500 μM).35 Despite the immunophenotypic heterogeneity between BLCLs, these TCR-like mAbs were capable of detecting endogenous expression of EBV latency proteins as the common denominator among these cells. Importantly, these TCR-like mAbs bound to EBV-transformed BLCLs, but not to PBMCs, from which they were derived, suggesting these antibodies might target only cells that are constantly expressing high levels of the viral antigens of interest. A single weekly injection of these antibodies in vivo decreased tumor load within the organs of engrafted NSG mice. Interestingly, although E1 did not possess the best binding affinity profile, E1 had the greatest number of surface targets bound and the best treatment efficacy data. This suggested that binding avidity might also be an important contributing factor to the overall performance of therapeutic antibodies, and that focus should be placed on generating TCR-like mAbs targeting well-characterized immunodominant epitopes. The balance between affinity and avidity might also explain why L1 and L2, despite showing better binding affinities and being capable of reducing tumor burden in vivo, could not confer survival advantages. Indeed, the treatment efficacy of trastuzumab/herceptin has been correlated with the density of its epitope HER2 on breast cancer cells.36 Thus, the in vivo efficacy of E1 could be attributed to the greater availability of surface HLA-A2/EBNA1 on BLCLs.
Early findings suggested that EBNA1 could inhibit its own presentation.37,38 However, subsequent studies have demonstrated the ability to detect EBNA1-specific CD8+ T cells, and that EBNA1 could be presented on MHC class I via antigen processing that involved protein synthesis than degradation.39-41 Using E1, we have similarly reported the direct detection of a high number of HLA-A2/EBNA1 complexes on nasopharyngeal carcinoma biopsies and EBV-positive cell lines.13 These findings supported our current observation that EBNA1 could indeed be presented endogenously by BLCLs. Notably, because EBNA1 is expressed in both lytic and latent EBV infections and is present across all EBV malignancies, E1 might potentially be applied to latency I- and latency II-associated diseases.
There has been growing interest in targeting EBV-associated diseases in a tumor-specific manner through immunotherapy.42 The treatment efficacy of rituximab suggests that mAbs can indeed be used to treat PTLD.2-4 The cross-reactivity and depletion of healthy B cells is, however, counterproductive as humoral responses are important for long-term protective immunity. Studies exploring the therapeutic usage of EBV-specific cytotoxic T lymphocytes in PTLD has delivered encouraging results.43-47 Still, this approach is limited by the prolonged, labor-intensive culturing time involved and the difficulty of achieving cell numbers sufficient to treat a large number of patients.42 Furthermore, possible donor-derived pathogens and the cost of cellular therapy should be thoroughly considered. The anti-EBV TCR-like mAbs described here represent a combination of both entities by their capacity to target tumor cells in a pMHC-specific manner and to carry out antibody-mediated effector functions. These mAbs can potentially be manufactured on a large-scale basis for greater availability and outreach. These mAbs may also carry out antitumor activity that is not limited by the immunosuppressive microenvironments associated with tumor sites and would be particularly useful for transplant recipients whose immune systems are suppressed and who have inadequate effector functions. Conjugation of these mAbs with cytokines or immunotoxins may also enhance their cytotoxicity against targeted tumors. Administering a combination of the 3 TCR-like mAbs may result in synergistic effects and may reduce the likelihood of developing antigen-negative tumor variants, but this has not been addressed in our current study.
Devoid of a functional adaptive immune system, NSG mice can be engrafted with BLCLs without much impediment, thus providing a simulation of immunosuppressed patients with B-cell lymphoproliferation in PTLD. As BLCLs are heterogeneous and unique to individuals, the xenograft model used here may provide a customized preclinical model for predicting the therapeutic efficacy of the anti-EBV TCR-like mAbs.
Antibodies mediate effector functions through several modes of action, including direct killing, antibody-dependent cellular cytotoxicity, complement-dependent cytotoxicity, and phagocytosis.14 We suggested here that mAbs (ie, E1) could perform dual functionalities, and in particular, the induction of phosphatidylserine on the target cell surface and the enhancement of Fc-dependent phagocytosis of these target cells. This also indicated that effector mechanisms of mAbs might not always be strictly immune-dependent or independent, and that mAbs that can mediate more than 1 effector mechanism may provide a greater therapeutic effect. As studies were performed in NSG mice, we cannot at this point exclude the possibility that these anti-EBV TCR-like mAbs may perform other effector functions if the relevant immune cell types were to be present.
To date, several TCR-like mAbs have been generated to target viral epitopes in a HLA-restricted manner.10-12 Most of these mAbs have been used to investigate antigen presentation in the context of viral infections, and the treatment applications of TCR-like mAbs have been limited to those involving overexpressed tumor-associated antigens.14-19 Few have examined the therapeutic usage of viral epitope-specific TCR-like mAbs; we have demonstrated here that pathogen-derived antigens, when targeted on the basis of their surface display as pMHC complexes, can be prospective candidates for virus-associated cancers.
Therapeutics development in the recent years has trended toward immunotherapy. As a novel class of immunotherapeutics, the anti-EBV TCR-like mAbs we reported here have demonstrated high binding specificities and affinities, the ability to recognize endogenous targets on several BLCLs, and the capacity to carry out antibody-mediated effector functions, as well as to induce in vivo reduction of tumor burden. Taken together, our data provide evidence on the potential use of anti-EBV TCR-like mAbs in EBV-PTLD treatment.
Presented in part in abstract form at the Cancer Research Institute–Association for Cancer Immunotherapy–European Academy of Tumor Immunology–American Association for Cancer Research Inaugural International Cancer Immunotherapy Conference, New York, NY, September 18, 2015.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Min Zin Oo and Kar Wai Tan for their feedback on the study.
This work is supported by grants from Office of the Deputy President (Research & Technology), National University of Singapore WBS-R-182-000-192-133 and National Research Foundation Singapore R182-000-272-281 (P.A.M.) and by a grant from National Medical Research Council Singapore (CBRG/0064/2014) (N.R.J.G.). J.L. and J.A.L.C. were supported by Yong Loo Lin School of Medicine National University of Singapore Graduate Research Scholarships. W.J.T. was supported by the Singapore-MIT Alliance for Research and Technology Graduate Research Scholarship.
Authorship
Contribution: J.L. and W.J.T. designed and performed experiments, analyzed and interpreted data, prepared the figures, and wrote the manuscript; C.T.T., J.A.L.C., L.H.W., F.B.M., and Y.Z. provided reagents, performed experiments, and analyzed data; N.S. and A.P.C.L. provided reagents; N.R.J.G., B.J.H., S.H.C., and J.C. provided reagents and advice for experiments; and P.A.M. conceived the study, interpreted data, supervised the work, and prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paul A. MacAry, Centre for Life Sciences, 28 Medical Dr, National University of Singapore, Singapore 117456; e-mail: paul_macary@nuhs.edu.sg.
References
Author notes
J.L. and W.J.T. contributed equally to this study.