To the editor:
Survival of patients with sickle cell disease (SCD) in high-income countries has improved greatly in the last 60 years. In 1960, it was described as a “disease of childhood”1 whereas 25 years later, the Cooperative Study of Sickle Cell Disease reported that 85% of hemoglobin SS (HbSS) patients lived to adulthood. More recently, the estimate is 99% in London,2 97% in Paris,3 and 94% in the United States.4
Survival estimates have continued to improve; in 1994, the median survival for patients with HbSS/Sβ0 thalassemia was estimated at 42 to 48 years,5 increasing to 53 to 58 years in Jamaica in 20016 and 58 years in the United States in 2014.7 Nonetheless, the life expectancy of patients with SCD is still shortened by >2 decades compared with the general population.8-10
This study evaluates survival among adult patients with SCD followed at a single center in the United Kingdom. The study was an audit of clinical practice, and involved analysis of data collected in routine clinical care. All procedures followed were in accordance with the ethical standards of the Helsinki Declaration of 1975, as revised in 2008. Seven hundred twelve adult patients with SCD (16-80 years of age) at King’s College Hospital (London, United Kingdom) were observed over 10 years (2004-2013 inclusive) and mortality outcome was identified (5268 patient-years of observation; median, 8 years of observation per patient).
All patients, except for 1, were of African or African-Caribbean heritage. Of the 712 patients, 444 (62%) were HbSS, 229 (32%) were HbSC, 33 (5%) were HbSβ+ thalassemia, and 6 (1%) were HbSβ0 patients. For subanalysis, we considered HbSS and HbSβ0 thalassemia patients as a group. The median age for HbSS/Sβ0 patients was 32 years (interquartile range [IQR], 25-43 years); HbSC, 39 years (IQR, 29-48 years); and HbSβ+ thalassemia, 40 years (IQR, 31-58 years). α-Globin genotypes were available in 542 patients (76%) of which 62% were αα/αα, 32% αα/α−, and 5% α−/α− genotypes. During the study period, 72 patients (all HbSS) had received hydroxyurea therapy, and 71 patients had received regular blood transfusion. We underline the low uptake of hydroxyurea therapy in our cohort. Oxygen saturations by pulse oximetry and laboratory data collected during outpatient clinic attendance were documented. Laboratory results were averaged over the 10-year period to create a “steady-state” value for each patient. The mean number of hospital admissions under hematology for each patient was calculated from the total admissions/number of observed years of admissions. Local hospitals were contacted to identify outcome in patients not seen in 2012 or 2013; despite this, 104 (14.6%) were not reviewed in 2012 or 2013. Data collection finished on July 31, 2015.
IBM SPSS Statistics 22 was used for statistical analyses. Continuous variables were log-transformed where necessary to obtain normalized distributions. Kaplan-Meier survival analysis considered nonfatal cases as censored at their last clinic visit. Univariate Cox regression analysis was undertaken for the HbSS/Sβ0 subgroup only to identify risk factors for mortality. Dichotomous variables were handled as follows: α-thalassemia, default = no; sex, default = male; fetal hemoglobin (HbF) based on median split of validated HbF values, default HbF <5.5%; iron overload based on ferritin >1000 µg/L, default = no; mean hospitalization rate, default ≤0.5 admissions per year. We chose this cutoff based on the very skewed data distribution: it is clinically meaningful (equivalent to 1 admission every 2 years) and to ensure we had large enough numbers in the “high admission rate” group for statistical analyses.
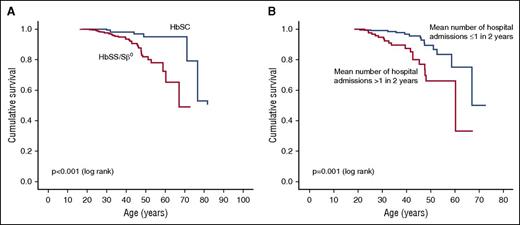
During the study period, 43 of the 712 patients (6.0%) died at a median age of 42 years (IQR, 31-48 years). They included 33 deaths in the 450 HbSS/HbSβ0 group (7.3%), at a median age of 41 years (IQR, 30-47 years), and 8 deaths in 229 HbSC patients (3.5%) at a median age of 46 years (31-72 years). For the HbSS/HbSβ0 group, Kaplan-Meier analysis gave an estimated median survival of 67 years (confidence interval [CI], 55-78 years), significantly lower than in HbSC (P < .001; Figure 1A). For HbSS/HbSβ0, there was a 90% estimated survival to 45 years (39-51 years), 80% to 51 years (CI, 44-57 years), and 70% to 60 years (CI, 51-69 years).
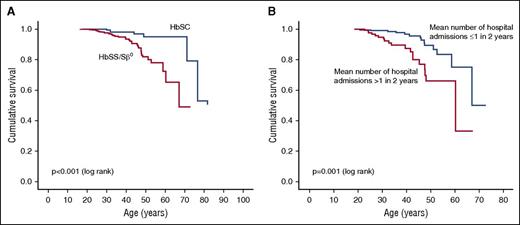
Kaplan-Meier survival curves. (A) Survival curve by sickle genotype. (B) Survival curve for HbSS/Sβ0, by hospitalization frequency.
Kaplan-Meier survival curves. (A) Survival curve by sickle genotype. (B) Survival curve for HbSS/Sβ0, by hospitalization frequency.
Subanalysis was undertaken for the HbSS/HbSβ0 subgroup; the sample size in the HbSC subgroup was too small. Median survival in patients with high hospital admission rates (>0.5 admissions per year) was 60 years (CI, 43-77 years), significantly lower than that in patients with low admission rates (≤0.5 per year) (P = .001; Figure 1B).
Univariate Cox regression analysis (Table 1) revealed that neither α-thalassemia nor sex were significant risk factors for death. Lack of difference in survival between the sexes may be due to the low numbers of deaths. Hospitalization frequency was a simple but strong predictor of survival in SCD; the risk of death was more than threefold if patients had high-frequency admissions compared with those with low admission rate. Neither hydroxyurea nor blood transfusion was associated with mortality. This likely reflects both the relatively low use of these therapies in our cohort and also the disproportionate use of these therapeutic strategies in our younger patients, confounding the data. Risk of death was increased nearly threefold if baseline oxygen saturations were low (<95%).
For steady-state laboratory results, risk of death was increased if there was: increased white blood cell count, low baseline HbF level, higher lactate dehydrogenase, higher C-reactive protein, or iron overload (ferritin >1000 μg/L). The correlation of disease severity with iron overload is likely via transfusion rate; it is unclear whether iron overload in itself is an independent risk factor. For hepatic enzymes, risk of death was increased if total bilirubin, aspartate transaminase (AST), or alkaline phosphatase were raised, but neither alanine transaminase nor γ-glutamyl transferase affected mortality risk. This may reflect red cell rather than hepatic origin of bilirubin and AST. Conspicuously, AST provides more dramatic hazard ratios than lactate dehydrogenase as a marker of hemolysis. Both measures of renal dysfunction (creatinine and urinary albumin creatinine ratio) demonstrated significant associations with mortality.
Multivariate Cox regression analysis (Table 2) was based on combining variables associated with risk of death in the univariate analysis, plus sex and age at the start of the study. Variables that remained independently significant after multivariate analysis were high admission rate (>0.5 per year), Ln creatinine, and Ln aspartate transaminase, each associated with striking hazard ratios (Table 2), suggesting that poor renal function, excess hemolysis, and frequent hospital admissions can all contribute independently to mortality risk in SCD.
In this retrospective analysis, we have demonstrated a high estimated survival (median, 67 years) for adults with HbSS/HbSβ0 at a single UK center, which is markedly higher than recent estimates from other institutions. We speculate the reasons: close monitoring of patients in a specialist hematology clinic, plus regular joint care with other specialists (renal, hepatology, neurology, cardiology, obstetrics, and orthopedics); inpatient management by a dedicated health-care team; on-site erythrocytapheresis; and a focused “transition program” to ensure safe transition of teenagers to the adult service. Four of the 43 deaths were in patients under the age of 25 years: 1 from hemopericardium due to stab wound, 1 from cerebral hemorrhage, and 2 from fulminant hepatic failure. We did not assess the socioeconomic class of each patient, but they were from a broad spectrum of social backgrounds. All of these features are similar to other large sickle centers in the United Kingdom.
We acknowledge some study limitations. As an adult-only study, exclusion of pediatric patients may have inflated survival estimates; however, the vast majority of SCD patients reach adulthood in the United Kingdom.2 We concede that we did not model for those “lost in transition” between pediatric and adult care. However, all 100 patients who turned 19 years of age in 2008 to 2013 inclusive (data from the King’s Pediatric Sickle database) have been seen in the adult clinic. We also recognize some missing data for those not reviewed at the end of the study period, despite repeated attempts to obtain information. We also acknowledge the low uptake of hydroxyurea in our cohort (72 of 450 of HbSS/HbSβ0 patients).
Although life expectancy for a patient with SCD in the United Kingdom continues to improve, it still falls behind that in the general population in London, where it is 80.3 years for men, and 84.2 years for women.11 We confirmed known predictors of mortality in SCD including markers of cardiorespiratory dysfunction, renal impairment, and hemolysis as well as frequent hospitalization rate.5,6,12-14 Although these risk factors are not causative, they certainly contribute to the mortality and morbidity in SCD. These risk factors identify higher risk patients who perhaps should be prioritized for therapies including hydroxyurea and hematopoietic stem cell transplantation.
Authorship
The current affiliation for S.L.T. is Sickle Cell Branch, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD.
Acknowledgments: The authors thank Clive Stringer (King’s College Hospital) for the help in data extraction from the Electronic Patient Record system (EPR). The authors thank David Rees and Sandra O’Driscoll from the King’s College Hospital Pediatric Sickle Service for data on pediatric patients.
This work was supported by the Medical Research Council (MRC) UK (MRC nos. G0001249 and ID62593) (S.L.T.). A.D. acknowledges financial support from the National Institute for Health Research (NIHR) Biomedical Research and from the NIHR Collaboration for Leadership in Applied Health Research and Care South London at King’s College Hospital National Health Service (NHS) Foundation Trust.
The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the UK Department of Health.
Contribution: K.G. and S.L.T. designed the research study; K.G., E.D., and M. Allman collected data; K.G., E.D., M. Allman, A.M., M. Awogbade, and S.L.T. provided patient care and follow-up; K.G. and A.D. analyzed the data; K.G. and S.L.T. wrote the paper; and all authors participated in editing the final version of paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Swee Lay Thein, Sickle Cell Branch, National Heart, Lung and Blood Institute, National Institutes of Health, Building 10-CRC, Room 5E-5142, 10 Center Dr, Bethesda, MD 20892; e-mail: sl.thein@nih.gov; and Kate Gardner, Molecular Haematology, King’s College London, James Black Centre, 125 Coldharbour Ln, London SE5 9NU, United Kingdom; e-mail: kate.gardner@doctors.org.uk.