To the editor:
Graft-versus-host disease (GVHD), both acute and chronic, is still a leading cause of nonrelapse mortality after transplantation.1 High-dose, posttransplantation cyclophosphamide (PTCy) is an attractive approach for in vivo allodepletion across the HLA barrier in allogeneic hematopoietic stem cell transplantation (HSCT), aimed at inducing a state of immunologic tolerance and preventing GVHD.2 This clinical platform was demonstrated first in the reduced-intensity conditioning, haploidentical allogeneic HSCT setting3,4 and later as single-agent GVHD prophylaxis after myeloablative conditioning and HLA-matched-related or -unrelated bone marrow allografting.5 Moreover, we have recently reported encouraging results in a haploidentical setting with the combination of PTCy and sirolimus as GVHD prophylaxis (Sir-PTCy),6 with a potential reduced transplant-related mortality (TRM) and GVHD incidence over a GVHD prophylaxis based on sirolimus–antithymocyte globulin.7
PTCy has been widely investigated in in the haploidentical context, with bone marrow as a source and in association with calcineurin inhibitors (CNIs),4,8-10 but limited series have been reported after HLA-matched HSCT with peripheral blood as a source or CNI-free GVHD prophylaxis.11 Mielcarek et al recently reported their experience on PTCy followed by cyclosporine in HLA-matched mobilized blood cell grafts.12,13 Here, we describe the use of PTCy and sirolimus as GVHD prophylaxis after HLA-matched peripheral blood HSCT, aiming to increase its feasibility by reducing TRM, as recently obtained by our group in the haploidentical setting.6
We report 28 consecutive patients, treated from 2014 to 2016, according to a Sir-PTCy6 GVHD prophylaxis schedule with an allogeneic HSCT from a matched-related donor (MRD; n = 15) or a matched-unrelated volunteer donor (MUD; n = 13). All patients were treated according to current institutional programs upon written informed consent for transplant procedures.
HLA compatibility among donor-recipient pairs was assessed by 10 loci molecular typing (HLA-A, -B, -C, -DRB1, -DQB1) at the allelic level; MUDs were matched at 10 of 10 loci in 11 patients and 9 of 10 loci in 2 patients. Comorbidities were evaluated according to the Comorbidity-Age Index.14 Immune reconstitution was evaluated by flow cytometry and analyzed with FCS Express software (De Novo Software). Neutrophil engraftment was defined as achievement of an absolute neutrophil count >500 cells/mm3 for 3 consecutive days. Acute and chronic GVHD were defined and scored according to the Glucksberg and National Institutes of Health consensus criteria, respectively.15-17 TRM was defined as death from any cause without evidence of relapse or progression of the original disease. Progression-free survival (PFS) was defined as the interval from HSCT to either relapse or progression or death in remission (whichever came first). Overall survival (OS) was defined as the interval from HSCT to death from any cause. The probabilities of PFS and OS were estimated using the Kaplan-Meier estimator. Cumulative incidences (CIs) were estimated for engraftment, GVHD, TRM, and relapse to accommodate competing risks.18 Univariate comparisons of survival curves were made using the log-rank test, whereas the Gray test was used for univariate comparisons of CI functions.19 The type I error rate was fixed at 0.05 for determination of factors associated with time to event. Statistical analyses were performed with R (R Development Core Team, Vienna, Austria) software packages.
Patients, donors, and graft characteristics are provided in Table 1. Median age at HSCT was 46.5 years (range, 25-77 years) and 16 patients were males. The majority of patients were affected by acute leukemia (39%) and non-Hodgkin lymphoma (22%). A total of 17 patients (61%) were in hematologic remission at HSCT. According to the Disease Risk Index,20 the patients were stratified in very-high (n = 1), high (n = 14), or intermediate (n = 13) risk.
All patients received a myeloablative conditioning regimen, consisting of treosulfan (14 g/m2 per day) on days −6 to −4, fludarabine (30 mg/m2 per day) on days −6 to −2, and melphalan (70 mg/m2 per day) on days −2 and −1. One patient received thiotepa (5 mg/kg per day) on days −3 and −2, as a substitute for melphalan, targeting previous central nervous system disease involvement.
Graft source was peripheral blood stem cells (PBSCs), mobilized with subcutaneous granulocyte-colony-stimulating factor and collected by leukapheresis and infused without any ex vivo manipulation. Median CD34+ and CD3+ cell doses were 5 × 106/kg (range, 4-7) and 189 × 106/kg (range, 53-456), respectively.
Postgrafting immunosuppression consisted of PTCy (50 mg/kg per day) on days 3 and 4, followed by sirolimus (orally, monitored 2 times a week to maintain a target therapeutic plasma level of 5-14 ng/mL), withdrawn between months 3 and 6 after HSCT in absence of GVHD or relapse. Mycophenolate mofetil (10 mg/kg thrice daily orally) was added in patients receiving HSCT from MUD, and was withdrawn on day 30. Sodium 2-sulfanylethanesulfonate and IV hydration were administered for uroprotection.
All of the patients experienced a sustained donor cell engraftment. The median time to a neutrophil count of ≥500/μL was 23 days (range, 12-56 days). Post-HSCT recovery of lymphocyte subsets was broad and fast, with a median of 343 CD3+/mL, 178 CD4+/mL, and 183 CD8+/mL T cells at 30 days from HSCT. Cytomegalovirus (CMV) reactivation occurred in 7 patients, who received ganciclovir; 2 patients experienced a gut CMV disease. Invasive fungal diseases were reported in 6 cases (4 probable, 2 possible according to European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group Consensus Group).21 Only 3 patients developed sepsis from multidrug-resistant gram-negative bacteria.
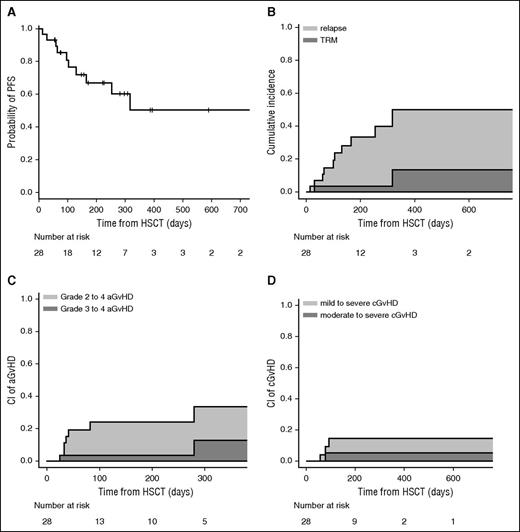
Clinical outcomes are shown in Figure 1. The CI of grades II-IV and III-IV acute GVHD at 100 days was 23% and 4%, respectively. The cumulative incidence of chronic GVHD was 13% at 1 year; we observed moderate or severe forms of chronic GVHD only in 4% of patients.
PFS, relapse, TRM, and GVHD. (A) Probability of PFS. (B) CI of relapse and TRM. (C) CI of grade II-IV acute GVHD (aGVHD) and grade III-IV aGVHD. (D) CI of mild to severe chronic GVHD (cGVHD) and moderate to severe cGVHD.
PFS, relapse, TRM, and GVHD. (A) Probability of PFS. (B) CI of relapse and TRM. (C) CI of grade II-IV acute GVHD (aGVHD) and grade III-IV aGVHD. (D) CI of mild to severe chronic GVHD (cGVHD) and moderate to severe cGVHD.
The CIs of relapse and TRM were 36% and 14% at 1 year, respectively. In univariate analysis, OS, PFS, and grades II-IV acute GVHD and chronic GVHD were similar between MUD and MRD transplantation. The CI of relapse was higher in MRD transplantation (P = .007), whereas TRM was significantly higher in the MUD group (P = .01), probably due to higher pretransplant comorbidities. However, we did not find a statistically significant effect of Sorror comorbidity index or age on survival. Eight patients have died. The causes of death were relapse (n = 5), infection (n = 2), and GVHD (n = 1). With a median follow-up of 225 days (range, 56-841 days) for surviving patients, 2-year estimated OS was 64%. The composite end point of GVHD-free/relapse-free survival22 was 45% at 1 year, in which events include grade 3-4 acute GVHD, systemic therapy-requiring chronic GVHD, relapse, or death; 58% of surviving patients are off from all immunosuppression at 1 year.
Although limited by a small number of patients with relatively short follow-up, our study suggests that in MRD and MUD transplants, PTCy and sirolimus are active, with an acceptable nonrelapse mortality and low incidence of chronic GVHD, enabling the use of unmanipulated peripheral blood grafts. The selective nature of cyclophosphamide activity permits rapid immune reconstitution, reducing the risk of severe infections. Moreover, HLA-matched allogeneic HSCT from PBSC grafts using double alkylating chemotherapy, PTCy, and sirolimus is associated with relevant survival in high-risk diseases.
Our results confirms the data reported by Mielcarek et al,12 in the context of a CNI-free GVHD prophylaxis. Sir-PTCy is a potential option in HLA-matched PBSC HSCT, warranting prospective comparative trials with the standard use of antithymocyte globulin and CNI as GVHD prophylaxis in unmanipulated MRD and MUD grafts.23,24
Authorship
Contribution: R.G., F.L., J.P., and F.C. designed the study, analyzed the data, and wrote the manuscript; and all authors contributed to patient clinical care and data collection and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Fabio Ciceri, Hematology and Bone Marrow Transplantation Unit, University Vita-Salute San Raffaele, IRCCS San Raffaele Scientific Institute, Milan, Italy; e-mail: ciceri.fabio@hsr.it.