Key Points
In vivo Treg effect depends on TNFα produced by T cells.
TNF/TNFR2 interaction represents a novel immune checkpoint therapy to modulate alloreactivity after allo-HCT.
Abstract
Therapeutic CD4+Foxp3+ natural regulatory T cells (Tregs) can control experimental graft-versus-host disease (GVHD) after allogeneic hematopoietic stem cell transplantation (allo-HCT) by suppressing conventional T cells (Tconvs). Treg-based therapies are currently tested in clinical trials with promising preliminary results in allo-HCT. Here, we hypothesized that as Tregs are capable of modulating Tconv response, it is likely that the inflammatory environment and particularly donor T cells are also capable of influencing Treg function. Indeed, previous findings in autoimmune diabetes revealed a feedback mechanism that renders Tconvs able to stimulate Tregs by a mechanism that was partially dependent on tumor necrosis factor (TNF). We tested this phenomenon during alloimmune response in our previously described model of GVHD protection using antigen specific Tregs. Using different experimental approaches, we observed that control of GVHD by Tregs was fully abolished by blocking TNF receptor type 2 (TNFR2) or by using TNF-deficient donor T cells or TNFR2-deficient Tregs. Thus, our results show that Tconvs exert a powerful modulatory activity on therapeutic Tregs and clearly demonstrate that the sole defect of TNF production by donor T cells was sufficient to completely abolish the Treg suppressive effect in GVHD. Importantly, our findings expand the understanding of one of the central components of Treg action, the inflammatory context, and support that targeting TNF/TNFR2 interaction represents an opportunity to efficiently modulate alloreactivity in allo-HCT to either exacerbate it for a powerful antileukemic effect or reduce it to control GVHD.
Introduction
Acute graft-versus-host disease (GVHD) is one of the major causes of morbidity and mortality following allogeneic hematopoietic stem cell transplantation (allo-HCT). In murine models, cell therapy using CD4+CD25highFoxp3+ regulatory T cells (Tregs) was shown to efficiently prevent GVHD1-3 without hampering immune reconstitution4 or the graft-versus-leukemia (GVL) effect.5,6 The efficacy and reproducibility of this therapy in preclinical models over the past 15 years by different groups in different mouse models using murine or human polyclonal or antigen-specific Treg cells led to the development of clinical trials with promising preliminary results.7-9 The mechanisms enabling this action rely on the ability of Tregs to directly act on conventional T cells (Tconvs).4-6,10 Here, we hypothesize that as Tregs are capable of modulating Tconv response, likewise Tconvs also modulate Treg action, resulting in an effective feedback mechanism.
Tumor necrosis factor (TNF) constitutes a likely candidate for mediating the effect of Tconvs on Tregs for several reasons: (1) This cytokine is abundantly produced during the cytokine storm following allo-HCT.11 (2) In the setting of autoimmunity, TNF was previously shown to boost proliferation and suppressive activity of Tregs by signaling through its type 2 receptor (TNFR2).12-15 This was observed in mice and humans, both in healthy individuals and type 1 diabetes patients.16-18 (3) Tregs express higher levels of TNFR2 compared with Tconvs, and Tregs expressing the highest level of TNFR2 are most suppressive in mice and humans.12,13,15
The role of TNF/TNFR2 interaction in the control of GVHD by Tregs has not been studied. Addressing this question may help us to better understand the physiopathology of the disease. It is also relevant in regard to anti-TNF treatment that is sometimes administered to patients with severe GVHD. The activation of TNF receptor type 1 (TNFR1) could be associated with increased inflammation and tissue damage in allo-HCT19 and blocking this pathway could ameliorate GVHD by reducing cell death of gastrointestinal cells. Thus, one may hypothesize that anti-TNF treatment would have a beneficial effect by blocking TNFR1 triggering but a detrimental effect by blocking TNFR2 triggering on Tregs, the latter phenomenon being assessed in this work. Here, we explored the hypothesis that in the context of Treg-based therapy of GVHD, Tconvs regulate Tregs via TNFR2 signaling. Using 3 different experimental approaches to prevent TNF/TNFR2 interaction, we showed that the suppression of GVHD obtained by transfer of Tregs strongly depended on TNFR2 expressed by Tregs and TNF produced by Tconvs.
Methods
Mice
Wild-type (WT) C57BL/6 (B6 H-2b) and B6C3HF1 (H-2kxb) mice were purchased from Harlan Laboratories (Gannat, France) and Charles River Laboratories (Saint-Germain-Nuelles, France). TNF−/− and TNFRs1b−/− (TNFR2−/−) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). All mice were on a C57BL/6 background. Mice were housed under specific pathogen-free conditions. All experimental protocols were approved by the local ethics committee (authorization number 11/12/12-5B) and are in compliance with European Union guidelines.
Treg preparation
Tregs were prepared as previously described.20 Briefly, spleens and lymph nodes from C57BL/6 female mice were collected and mechanically dilacerated. Cell suspension was stained with biotin-coupled anti-CD25 monoclonal antibody (mAb) (7D4; BD Biosciences, San Diego, CA), followed by anti-biotin microbeads (Miltenyi Biotec, Paris, France), and CD25+ cells were positively selected through magnetic large selection column (Miltenyi Biotec). Selected cells were stained with the following mAb’s: CD4–fluorescein isothiocyanate (FITC; eBioscience, San Diego, CA), CD62L–phycoerythrin (PE; eBioscience), CD25-biotin (BD Biosciences), and streptavidin-PE-Cy5 (eBioscience). CD4+ CD25high CD62Lhigh cells were then sorted using a MoFlo Legacy (Beckman Coulter, Villepinte, France), with a purity of 99%. For HY-Treg preparation, cells were cultured for 3 to 4 weeks in the presence of recombinant murine interleukin-2 (IL-2) (10 ng/mL; PeproTech, Neuilly-sur-Seine, France) and weekly stimulated with CD8+ dendritic cells previously loaded with the HY peptide (10 µg/mL, N-15-S, NY; PolyPeptide, Strasbourg, France) in the presence of granulocyte-macrophage colony-stimulating factor (20 ng/mL; PeproTech). CD8+ dendritic cells were isolated from splenocytes of C57BL/6 mice, as previously described.20 For recipient-specific (rs)–Treg preparation, cells were cultured for 3 to 4 weeks in the presence of recombinant murine IL-2 and weekly stimulated with irradiated total splenocytes from C3H female mice, as previously described.6,10
GVHD and transplantation models
Eight- to 12-week-old recipient B6C3HF1 female mice received a 10 Gy irradiation followed by retro-orbital infusion of 10 × 106 bone marrow (BM) cells + 2 × 106 CD3+ T cells, with or without HY-Treg cells in a 1:1 ratio (ie, 2 × 106 HY-Treg cells). BM and T-cell suspensions were prepared using leg bones and splenocytes, respectively, as previously described. All infused cells were isolated from female C57BL/6 mice (semi-allogeneic model). As recipient and donor mice were females, HY-Treg cells were activated in vivo by repeated retro-orbital infusions of 100 μg of the HY peptide (at D0, D1, D3, and D6), as previously described.20 For rs-Treg experiments, mice were transferred with rs-Treg cells in a 1:1 ratio.
Antibody treatment
Anti-TNFR2 (TR75-54.7) mAb was purchased from Bio X Cell (West Lebanon, NH). Recipient mice were treated with 3 intraperitoneal injections of 500 μg of the antibody on days 0, 2, and 4.
GVHD clinical grading
GVHD clinical score was calculated 2 to 3 times per week. Each of the 5 following parameters was scored 0 (if absent) or 1 (if present): weight loss >10% of initial weight, hunching posture, skin lesions, dull fur, and diarrhea. Dead mice received a global score of 5. Mice were euthanized in case of weight loss >30% of initial weight or maximal clinical grade (ie, 5/5).
Histopathological examination
Livers, lungs, skin, and small and large bowel samples were preserved in Bouin's fixative and embedded in paraffin. For these organs, 5-µm-thick sections were stained with hematoxylin and eosin for histological examination as previously described.6,10 Briefly, 1 pathologist analyzed slides in a blinded fashion to assess the intensity of GVHD. GVHD lesions in each sample were scored according to a semiquantitative scoring system described by Hill et al with minor modifications.21
Flow cytometry
Two weeks after transplantation (ie, at D13, D0 being the date of transplantation and Treg cell injection), recipient mice were euthanized and their spleens collected. Because of the low proportion of Treg cells among splenocytes and the low overall spleen cellularity at D13, cell suspensions obtained for each spleen were enriched in CD4+ and CD8+ cells after labeling with anti-CD4 and anti-CD8 microbeads (Miltenyi Biotec) and positive magnetic selection through large selection columns (Miltenyi Biotec). Selected cells were then stained with the following mAb’s: CD4-FITC, CD4-allophycocyanin and CD4-Vioblue (Miltenyi Biotec), Foxp3-PE-Cy5 and Foxp3-V450 (eBioscience), CD25-PE-Cy7 (eBioscience), CD62L-PE (eBioscience), inducible T-cell costimulator (ICOS)–PE (eBioscience), cytotoxic T lymphocyte associated protein 4 (CTLA4)–biotin (followed by streptavidin-PE-Cy7; eBioscience), interferon γ (IFNγ)–PE (Miltenyi Biotec), TNFα-FITC (Miltenyi Biotec), and CD8α-FITC (eBioscience). Intracellular Foxp3 staining was performed according to the manufacturer’s instructions, using the Foxp3 staining buffer set from eBioscience. For intracellular cytokine staining, cells were restimulated with 1 μg/mL PMA (Sigma Aldrich, Saint Quentin Fallavier, France) and 0.5 μg/mL Ionomicyn (Sigma Aldrich) for 5 h, in the presence of GolgiPlug (1 μL/mL; BD Biosciences). Events were acquired on a FACSCanto II flow cytometer (BD Biosciences) and analyzed using FlowJo software vX.0.7 (FlowJo LLC, Ashland, OR).
Statistical analysis
Prism (GraphPad Software) was used for statistical analysis. Kaplan-Meier survival curves were compared using log-rank test. For analysis of GVHD clinical grading curves, area under curve (AUC) was calculated for each mouse, and then Student t test or 1-way analysis of variance (ANOVA) with post hoc analysis was performed depending on the number of comparatives. For cytometry analysis, we have normalized the mean fluorescence intensity (MFI) values with T-cell + Treg-cell control group. Then we used unpaired, 2-tailed Student t tests for generation of P values.
Results
TNFR2 plays a pivotal role in Treg-mediated prevention of GVHD
To assess the role of TNF on GVHD protection by Treg administration, we first used our recently described model in which the disease was prevented by transfer in female recipients of donor Tregs specific for the exogenous (ie, nondonor, nonrecipient) HY antigen at time of HCT, followed by their in vivo reactivation by HY peptide immunization.20 In a semi-allogeneic condition C57BL/6 → [B6xC3H]F1 of BM transplantation, GVHD protection at 1:1 Treg/Tconv ratio strictly depends on HY immunization. We evaluated the role of TNFR2 in Treg-mediated GVHD protection using a blocking anti-TNFR2 mAb (Figure 1A-B). Mice transferred with Tconvs developed severe GVHD that was prevented by the cotransfer of HY-Tregs. The beneficial effect of Treg administration was fully abolished in mice that were treated with the blocking anti-TNFR2 mAb. These latter mice displayed high clinical GVHD scores and decreased survival, as compared with HY-Treg-treated control mice. In order to assess whether a higher Treg/Tconv ratio could overcome the disadvantage because of TNFR2 blockade, we reproduced the same experiment doubling the number of HY-Treg infused in recipient mice (2:1 Treg/Tconv ratio). Even with this increased number of therapeutic Tregs, blocking TNFR2 fully abolished the Treg-dependent GVHD protection (supplemental Figure 1, available on the Blood Web site).
TNFα/TNFR2 disruption using anti-TNFR2 blocking mAb abolishes protective effect of Treg after allo-HCT. (A-B) [B6 x C3H]F1 female mice underwent total body irradiation (TBI) followed by transplantation with B6 BM cells plus T cells or with B6 BM cells plus T cells supplemented with HY-Tregs. HY peptide was administered at day 0, 1, 3, and 6, and mice were treated or not with anti-TNFR2 administered at day 0, 2, and 4. The experiment was performed twice, and the resulting survival (A) and clinical score (B) data were pooled and compared among the 3 groups of mice. (C-D) Experimental groups were constituted of mice grafted with B6 BM cells plus T cells treated or not with anti-TNFR2 administered at day 0, 2, and 4. The experiment was performed twice, and the resulting survival (C) and clinical score (D) data were pooled. Mice were euthanized in case of weight loss >30% of initial weight or maximal clinical grade (ie, 5/5). Kaplan-Meier survival curves were compared using log-rank test. For analysis of GVHD clinical grading curves, AUC was calculated for each mouse, and then Student t test or 1-way ANOVA with post hoc analysis was performed depending on number of comparatives. ns, nonsignificant. *P < .05; **P < .01; ***P < .001.
TNFα/TNFR2 disruption using anti-TNFR2 blocking mAb abolishes protective effect of Treg after allo-HCT. (A-B) [B6 x C3H]F1 female mice underwent total body irradiation (TBI) followed by transplantation with B6 BM cells plus T cells or with B6 BM cells plus T cells supplemented with HY-Tregs. HY peptide was administered at day 0, 1, 3, and 6, and mice were treated or not with anti-TNFR2 administered at day 0, 2, and 4. The experiment was performed twice, and the resulting survival (A) and clinical score (B) data were pooled and compared among the 3 groups of mice. (C-D) Experimental groups were constituted of mice grafted with B6 BM cells plus T cells treated or not with anti-TNFR2 administered at day 0, 2, and 4. The experiment was performed twice, and the resulting survival (C) and clinical score (D) data were pooled. Mice were euthanized in case of weight loss >30% of initial weight or maximal clinical grade (ie, 5/5). Kaplan-Meier survival curves were compared using log-rank test. For analysis of GVHD clinical grading curves, AUC was calculated for each mouse, and then Student t test or 1-way ANOVA with post hoc analysis was performed depending on number of comparatives. ns, nonsignificant. *P < .05; **P < .01; ***P < .001.
We then used TNFR2-deficient HY-specific Tregs, obtained from TNFR2–knockout (KO) mice, to confirm that the Treg control of GVHD by TNFR2 was mediated by TNFR2 expression by Tregs. Whereas TNFR2-sufficient control Tregs fully protected from GVHD, TNFR2-deficient Tregs completely failed to prevent the disease (Figure 2). Survival of the mice and clinical scores of GHVD were identical in mice receiving donor Tconvs alone and mice receiving donor Tconvs and TNFR2-deficient Tregs.
TNFα/TNFR2 disruption using TNFR2-KO Tregs abolishes the protective effect of Treg after allo-HCT. [B6 x C3H]F1 female mice underwent TBI followed by transplantation with B6 BM cells plus T cells or with B6 BM cells plus T cells supplemented with HY-Tregs produced from WT B6 or from TNFR2-deficient mice in order to prevent GVHD. HY peptide was administered at day 0, 1, 3, and 6. The experiment was performed twice, and the resulting survival and clinical score data were pooled. Kaplan-Meier survival curves (A) and curves of evolution of GVHD clinical score (B) over time were compared between the 3 groups of mice. *P < .05; **P < .01; ***P < .001.
TNFα/TNFR2 disruption using TNFR2-KO Tregs abolishes the protective effect of Treg after allo-HCT. [B6 x C3H]F1 female mice underwent TBI followed by transplantation with B6 BM cells plus T cells or with B6 BM cells plus T cells supplemented with HY-Tregs produced from WT B6 or from TNFR2-deficient mice in order to prevent GVHD. HY peptide was administered at day 0, 1, 3, and 6. The experiment was performed twice, and the resulting survival and clinical score data were pooled. Kaplan-Meier survival curves (A) and curves of evolution of GVHD clinical score (B) over time were compared between the 3 groups of mice. *P < .05; **P < .01; ***P < .001.
We then assess the role of TNFR2 in Treg-mediated protection in another transplant setting. Tregs naturally present in the donor T-cell inoculum were present in sufficient number to attenuate GVHD because their depletion accelerated the disease.1-3 Here, we observed that blocking TNFR2 led to a similar GVHD aggravation. Indeed, in mice grafted with BM cells and whole T cells containing Tregs at physiological level, administration of the blocking anti-TNFR2 mAb induced an accelerated GVHD (Figure 1C-D). Histological analyses performed at day 14 confirmed the exacerbated GVHD in the liver (P < .001) and a trend to an increase of GVHD for the small intestine and the colon of anti-TNFR2–treated mice (supplemental Figure 2). Surprisingly, we observed a reduced GVHD in the skin of grafted animals after anti-TNFR2 mAb treatment. This could be explained by the distinct biological feature of cutaneous Tregs vs Tregs of other tissues, including a specific pattern of homing receptors as recently reviewed,22 which may modify their sensitivity to TNF/TNFR2 signaling.
The number of splenocytes collected at day 14 importantly varied between the 2 groups of mice. Whereas spleens of mice grafted with T cells contain 65.4 × 106 ± 2.2 cells, this number fell sharply to 12.3 × 106 ± 9.6 in mice treated with anti-TNFR2 mAb, probably reflecting an accelerated GVHD. We next evaluated the effect of blocking TNFR2 on cytokine production in the spleen of mice developing GVHD after transfer of WT donor T cells. Mice treated with the anti-TNFR2 mAb had an increase in IFNγ and TNFα production in both CD4 and CD8 donor T cells (Figure 3).
TNFα/TNFR2 disruption using anti-TNFR2 blocking mAb increases inflammatory cytokine production by donor CD4 and CD8 T cells. [B6 x C3H]F1 female mice underwent TBI followed by transplantation with B6 BM cells plus T cells treated or not with anti-TNFR2 administered at day 0, 2, and 4. Mice were euthanized, and donor CD4+ and CD8+ T cells were analyzed at day 14 posttransplantation in the spleen of grafted animals. Mean absolute numbers of splenocytes and percentage of CD4+ and CD8+ donor T cells are determined as well as intracellular IFNγ and TNFα production. Each plot represents a mouse; Student t test analysis was performed to compare the anti-TNFR2 mAb effect on T cells. *P < .05; **P < .01; ***P < .001.
TNFα/TNFR2 disruption using anti-TNFR2 blocking mAb increases inflammatory cytokine production by donor CD4 and CD8 T cells. [B6 x C3H]F1 female mice underwent TBI followed by transplantation with B6 BM cells plus T cells treated or not with anti-TNFR2 administered at day 0, 2, and 4. Mice were euthanized, and donor CD4+ and CD8+ T cells were analyzed at day 14 posttransplantation in the spleen of grafted animals. Mean absolute numbers of splenocytes and percentage of CD4+ and CD8+ donor T cells are determined as well as intracellular IFNγ and TNFα production. Each plot represents a mouse; Student t test analysis was performed to compare the anti-TNFR2 mAb effect on T cells. *P < .05; **P < .01; ***P < .001.
In order to reinforce the robustness of our observations, we used a third transplant setting consisting of infusing Tregs that were rendered specific for recipient-type alloantigen (namely, rs-Treg) instead of HY-Treg to prevent GVHD. Whereas GVHD was prevented by rs-Treg administration, this protective effect was fully abolished when using anti-TNFR2 mAb (Figure 4).
TNFα/TNFR2 disruption and its effect on GVHD do not depend on the antigen specificity of therapeutic Tregs. (A-B) [B6 x C3H]F1 female mice underwent TBI followed by transplantation with (1) B6 BM cells plus 2 × 106 T cells or (2) B6 BM cells plus 2 × 106 T cells supplemented with 2 × 106 rs-Tregs or (3) BM cells plus 2 × 106 T cells collected from TNFα-deficient mice supplemented with 2 × 106 rs-Tregs. HY peptide was administered at day 0, 1, 3, and 6, and mice were treated or not with anti-TNFR2 administered at day 0, 2, and 4. The resulting survival (A) and clinical score (B) data were compared among the 3 groups of mice. Mice were euthanized in case of weight loss >30% of initial weight or maximal clinical grade (ie, 5/5). Kaplan-Meier survival curves were compared using log-rank test. For analysis of GVHD clinical grading curves, AUC was calculated for each mouse, and then 1-way ANOVA with post hoc analysis was performed. *P < .05; **P < .01; ***P < .001.
TNFα/TNFR2 disruption and its effect on GVHD do not depend on the antigen specificity of therapeutic Tregs. (A-B) [B6 x C3H]F1 female mice underwent TBI followed by transplantation with (1) B6 BM cells plus 2 × 106 T cells or (2) B6 BM cells plus 2 × 106 T cells supplemented with 2 × 106 rs-Tregs or (3) BM cells plus 2 × 106 T cells collected from TNFα-deficient mice supplemented with 2 × 106 rs-Tregs. HY peptide was administered at day 0, 1, 3, and 6, and mice were treated or not with anti-TNFR2 administered at day 0, 2, and 4. The resulting survival (A) and clinical score (B) data were compared among the 3 groups of mice. Mice were euthanized in case of weight loss >30% of initial weight or maximal clinical grade (ie, 5/5). Kaplan-Meier survival curves were compared using log-rank test. For analysis of GVHD clinical grading curves, AUC was calculated for each mouse, and then 1-way ANOVA with post hoc analysis was performed. *P < .05; **P < .01; ***P < .001.
Thus, using 2 different approaches (anti-TNFR2 mAb and TNFR2-deficient Tregs) and different types of Tregs (Tregs of the T-cell inoculum, therapeutic HY-Tregs, or rs-Tregs), we demonstrate that the control of GVHD by Tregs is TNFR2 dependent.
TNF produced by Tconvs is critical for the control of GVHD by Tregs
TNF can be produced by multiple cell types after allo-HCT. Because donor T cells are key actors of GVHD and one of the major TNF producers,11 we evaluated whether GVHD prevention by Tregs would be maintained when using TNFα-deficient donor T cells collected from TNFα-deficient mice. We first compared GVHD in mice transferred with TNFα-deficient and TNF-sufficient whole donor T cells in the absence of additional therapeutic Tregs. Both groups developed a similar clinical GVHD (Figure 5C-D). However, histological and biological analyses performed at day 14 showed that mice receiving TNFα-deficient T cells developed a more severe GVHD compared with mice transferred with TNF-sufficient T cells. Histological scores in the liver and small intestine were higher in the former mice compared with the latter mice (supplemental Figure 3). Also, numbers of splenocytes were reduced in mice transferred with TNFα-deficient T cells compared with TNFα-sufficient T cells. This was associated with an increase in IFNγ production in both CD4 and CD8 donor T cells in mice receiving TNFα-deficient T cells (Figure 6).
TNFα/TNFR2 disruption using TNFα-KO donor T cells abolishes protective effect of Treg after allo-HCT. (A-B) [B6 x C3H]F1 female mice underwent TBI followed by transplantation with B6 BM cells plus T cells or with B6 BM cells plus T cells supplemented with HY-Tregs or B6 BM cells plus T cells collected from TNFα-deficient mice instead of WT B6 mice and supplemented with HY-Tregs. HY peptide was administered at day 0, 1, 3, and 6. The experiment was performed 4 times, and the resulting survival (A) and clinical score (B) data were pooled and compared among the 3 groups of mice. (C-D) Experimental groups were constituted of mice grafted with B6 BM cells plus T cells or T cells collected from TNFα-deficient mice instead of WT B6 mice. The experiment was performed twice, and the resulting survival (C) and clinical score (D) data were pooled. Mice were euthanized in case of weight loss >30% of initial weight or maximal clinical grade (ie, 5/5). Kaplan-Meier survival curves were compared using log-rank test. For analysis of GVHD clinical grading curves, AUC was calculated for each mouse, and then Student t test or 1-way ANOVA with post hoc analysis was performed depending on number of comparatives. *P < .05; **P < .01; ***P < .001.
TNFα/TNFR2 disruption using TNFα-KO donor T cells abolishes protective effect of Treg after allo-HCT. (A-B) [B6 x C3H]F1 female mice underwent TBI followed by transplantation with B6 BM cells plus T cells or with B6 BM cells plus T cells supplemented with HY-Tregs or B6 BM cells plus T cells collected from TNFα-deficient mice instead of WT B6 mice and supplemented with HY-Tregs. HY peptide was administered at day 0, 1, 3, and 6. The experiment was performed 4 times, and the resulting survival (A) and clinical score (B) data were pooled and compared among the 3 groups of mice. (C-D) Experimental groups were constituted of mice grafted with B6 BM cells plus T cells or T cells collected from TNFα-deficient mice instead of WT B6 mice. The experiment was performed twice, and the resulting survival (C) and clinical score (D) data were pooled. Mice were euthanized in case of weight loss >30% of initial weight or maximal clinical grade (ie, 5/5). Kaplan-Meier survival curves were compared using log-rank test. For analysis of GVHD clinical grading curves, AUC was calculated for each mouse, and then Student t test or 1-way ANOVA with post hoc analysis was performed depending on number of comparatives. *P < .05; **P < .01; ***P < .001.
TNFα/TNFR2 disruption using TNFα-KO donor T cells increases inflammatory cytokine production by donor CD4 and CD8 T cells. [B6 x C3H]F1 female mice underwent TBI followed by transplantation with B6 BM cells plus T cells or T cells collected from TNFα-deficient mice instead of WT B6 mice. Mice were euthanized, and donor CD4+ and CD8+ T cells were analyzed at day 14 posttransplantation in the spleen of grafted animals. Mean absolute numbers of splenocytes and percentage of CD4+ and CD8+ donor T cells are determined as well as intracellular IFNγ and TNFα production. Each plot represents a mouse; Student t test analysis was performed to compare mice transferred with WT or TNFα-deficient donor T cells. *P < .05; **P < .01; ***P < .001.
TNFα/TNFR2 disruption using TNFα-KO donor T cells increases inflammatory cytokine production by donor CD4 and CD8 T cells. [B6 x C3H]F1 female mice underwent TBI followed by transplantation with B6 BM cells plus T cells or T cells collected from TNFα-deficient mice instead of WT B6 mice. Mice were euthanized, and donor CD4+ and CD8+ T cells were analyzed at day 14 posttransplantation in the spleen of grafted animals. Mean absolute numbers of splenocytes and percentage of CD4+ and CD8+ donor T cells are determined as well as intracellular IFNγ and TNFα production. Each plot represents a mouse; Student t test analysis was performed to compare mice transferred with WT or TNFα-deficient donor T cells. *P < .05; **P < .01; ***P < .001.
We then assessed whether GVHD protection by therapeutic HY-specific Tregs was maintained when donor T cells were TNF deficient. Although the cotransfer of HY-specific Tregs protected from GVHD in mice receiving TNF-sufficient T cells, this beneficial Treg effect was entirely lost with TNFα-deficient donor T cells (Figure 5A-B). Actually, we even observed that mice grafted with TNFα-deficient donor T cells supplemented with Tregs experienced an even greater degree of GVHD (trends in an accelerated mortality) compared with mice receiving only TNFα-deficient donor T cells. The loss of GVHD protection by Tregs when TNF-deficient T cells were transferred was confirmed when therapeutic rs-Tregs were injected in place of HY-Tregs (Figure 4). Thus, TNFα produced specifically by donor T cells is required for the Treg suppressive effect in GVHD.
After allo-HCT, TNF/TNFR2 blockade reduces Foxp3 and activation marker expression on Tregs
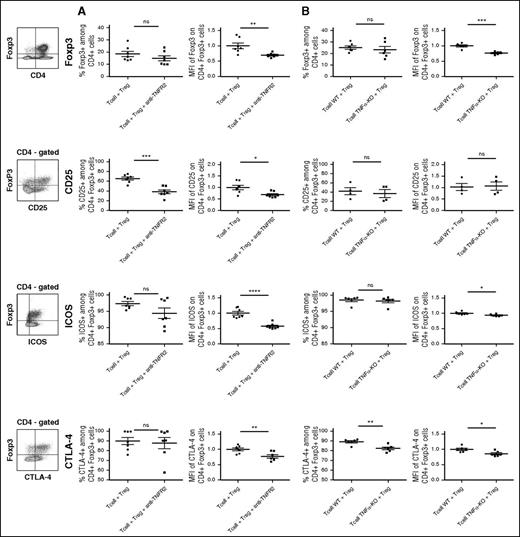
To analyze by what mechanism the control of GVHD by Tregs depends on TNF/TNFR2 interaction, we measured the proportion and activation markers of Tregs in the spleen collected at day 13 in mice grafted with HY-Tregs and either WT T cells treated with anti-TNFR2 mAb or TNF-deficient T cells. First, the expression level of Foxp3 was significantly reduced when TNF/TNFR2 interaction was inhibited in both settings, whereas Treg proportions remained unchanged (Figure 7A-B). This lower Foxp3 expression among whole Tregs was characterized by a reduced proportion of Foxp3high-expressing cells and an increased proportion of Foxp3int-expressing cells in both experimental models (supplemental Figure 4). A likely explanation would be that, in the absence of TNFR2 signaling in Tregs, Foxp3 would be downmodulated, suggesting that TNF stabilized Foxp3 expression in Tregs. We further analyzed the expression of CD25, the α chain of the IL-2 receptor constitutively expressed at the Treg cell surface membrane. We observed a dramatic decrease of the percentage of CD25+ cells and CD25 expression level among Tregs when anti-TNFR2 mAb was administered to grafted mice. Because in experimental allo-HCT CD25 expression is upregulated by IL-2,23 these results suggest that TNFα increased IL-2 responsiveness of Tregs in this context. Finally, we evaluated the expression of ICOS and CTLA4, which are important molecules in Treg biology. In both models of inhibition of TNF/TNFR2 interaction, ICOS and CTLA-4 expressions were reduced compared with controls, with a more pronounced effect in mice treated with the anti-TNFR2 mAb than mice grafted with TNFα-deficient T cells (Figure 7), probably reflecting the more complete abrogation of TNF signaling in the presence of the mAb.
Blockade of the TNFα/TNFR2 interaction reduces Foxp3 and activation markers expressions in Tregs used to prevent GVHD. GVHD experiments were reproduced using “anti-TNFR2” mAb treatment (A) or T cells collected from TNFα-deficient mice (B). Splenocytes from grafted animals were harvested at day 13 posttransplantation and enriched in CD4+ and CD8+ T cells through positive magnetic selection using large selection columns (Miltenyi Biotec). Depending on the marker evaluated, Tregs were stained with CD4-FITC, CD4-allophycocyanin or CD4-Vioblue, Foxp3-PE-Cy5 or Foxp3-V450, and CD25-PE-Cy7, ICOS-PE, CTLA4-biotin. Intracellular Foxp3 staining was performed using the Foxp3 staining buffer set from eBioscience. Cells were gated on CD4+ Foxp3+ T cells except for the percentage of Foxp3 (up), which is gated on CD4+ T cells. For each marker, the strategy of gating is indicated on the left of the figure. Each dot represents a single mouse. For each group of mice, horizontal lines represent mean value and standard error of the mean. MFI values are represented as ratio of the measured value for each sample to the mean value of the control group (ie, the group of mice receiving BM cells plus T cells and Treg cells). We have normalized the MFI values with T-cell + Treg control group. Then we used unpaired, 2-tailed Student t tests for generation of P values. *P < .05; **P < .01; ***P < .001; ****P < .0001.
Blockade of the TNFα/TNFR2 interaction reduces Foxp3 and activation markers expressions in Tregs used to prevent GVHD. GVHD experiments were reproduced using “anti-TNFR2” mAb treatment (A) or T cells collected from TNFα-deficient mice (B). Splenocytes from grafted animals were harvested at day 13 posttransplantation and enriched in CD4+ and CD8+ T cells through positive magnetic selection using large selection columns (Miltenyi Biotec). Depending on the marker evaluated, Tregs were stained with CD4-FITC, CD4-allophycocyanin or CD4-Vioblue, Foxp3-PE-Cy5 or Foxp3-V450, and CD25-PE-Cy7, ICOS-PE, CTLA4-biotin. Intracellular Foxp3 staining was performed using the Foxp3 staining buffer set from eBioscience. Cells were gated on CD4+ Foxp3+ T cells except for the percentage of Foxp3 (up), which is gated on CD4+ T cells. For each marker, the strategy of gating is indicated on the left of the figure. Each dot represents a single mouse. For each group of mice, horizontal lines represent mean value and standard error of the mean. MFI values are represented as ratio of the measured value for each sample to the mean value of the control group (ie, the group of mice receiving BM cells plus T cells and Treg cells). We have normalized the MFI values with T-cell + Treg control group. Then we used unpaired, 2-tailed Student t tests for generation of P values. *P < .05; **P < .01; ***P < .001; ****P < .0001.
Discussion
Here, we describe in the context of alloreactive immune responses a novel feedback mechanism by which Tconvs boost Treg activation. Indeed, we clearly demonstrate that the protection of GVHD by Tregs in allo-HCT strictly depends on TNF produced by effector T cells and TNFR2 expressed by Tregs. We believe our findings are solid because they rely on 3 different experimental approaches (blocking TNFR2 mAb, TNFR2-deficient Tregs, and TNFα-deficient Tconvs) and using 3 types of protective Tregs (polyclonal Tregs present at physiological level in the T-cell inoculum, therapeutic HY-Tregs, and therapeutic rs-Tregs). If a similar phenomenon exists in human, TNF-TNFR2 interaction would be critical in the control of GVHD by Tregs in allo-HCT performed either in routine or in clinical trials when therapeutic Tregs are injected.
These findings are in accordance with recent reports showing that, in the context of autoimmune diabetes, effector T-cell activation was able to boost Treg cell expansion, favoring disease control, a phenomenon partly dependent on TNF.14-16,24 Our results extend, in an allo-HCT setting, the observation that Tconvs exert a powerful boost of Treg activity. We also demonstrate for the first time that the sole defect of TNF production by donor T cells was sufficient to completely abolish the Treg suppressive effect in GVHD. Our findings expand our understanding of the diverse and complex effects of inflammation on Treg biology. Indeed, it was shown that some inflammatory factors, such as IL-6 or IL-4, promoted Foxp3 downmodulation and Treg instability.25,26 To the contrary, our study suggests that another inflammatory factor, TNF, maintained a high level of Foxp3 in Tregs in an inflamed environment. This is consistent with previous data in the context of autoimmune diseases in mice16 and with our recent findings that TNF increased Foxp3 expression in human Tregs in vitro.27
These results shed light on the conflicting data regarding the effect of anti-TNF drugs. These agents are widely used in the treatment of various autoimmune diseases. However, they are not efficient in all patients, and there are numerous reports describing paradoxical exacerbation of the initial disease or the occurrence of new autoimmune syndromes.28-31 Similarly, a number of clinical studies failed to demonstrated efficacy for anti-TNF treatment in preventing or treating GVHD.32,33 Our results support that the potential benefit of anti-TNF drugs in GVHD patients may also be counteracted by the inhibition of Treg function, thus promoting a vigorous alloreactive T-cell response.
Finally, we believe that our results pave the way for a novel immune checkpoint therapy to modulate alloreactivity after allo-HCT. Indeed, the TNF/TNFR2 signaling pathway represents a potential target for modulating alloreactivity. We previously observed that ex vivo Treg depletion from donor lymphocyte infusions could improve the GVL effect.34,35 TNF inhibition could also potentiate the GVL effect of donor lymphocyte infusions by blocking Treg effect with a much more simple and direct procedure. On the other hand, the alloreactivity could be strongly reduced to control GVHD by using TNFR2 agonist molecules in order to expand Tregs ex vivo.17 Indeed, it was recently observed that serum of recipient animals during acute GVHD induced Treg activation and enhanced their suppressive function through a TNF-dependent mechanism without inhibiting the GVL effect.36 These 2 aspects remain to be confirmed both in experimental GVHD and in xeno-GVHD induced by human cells. This represents a potential therapeutic strategy to tailor the intensity of the alloreactive response to the particular need over the course of the patient disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are indebted to Dan Avi Landau for critical reading of the manuscript.
M.L. was supported by a “poste d'accueil Inserm,” S.N. by the “région Ile de France, DIM Biothérapie,” and the département hospitalo-universitaire “Virus Immunité Cancer.” This work was supported by Association Française contre les Myopathies, Agence de la Biomédecine, Association de Recherche contre les hémopathies malignes, Fondation pour la recherche médicale (Victor et Erminia Mescle price) et Fondation Ramsay Générale de Santé (Cellular Therapy and Regenerative Medicine price).
Authorship
Contribution: M.L., S.N., B.L.S., and J.L.C. conceived the study and wrote the manuscript; M.L., S.N., C. Pilon, C.D., Y.B., and F.C. performed experiments; and M.L., S.N., C. Pilon, A.T., G.H.M., C. Pouchy, F.C., S.M., B.L.S., and J.L.C. analyzed the data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: José L. Cohen, Hôpital Henri Mondor, 53 Av du Mal de Lattre de Tassigny, 94010 Créteil, France; e-mail: jose.cohen@inserm.fr.
References
Author notes
M.L. and S.N. contributed equally to this study and are joint first authors.

![Figure 1. TNFα/TNFR2 disruption using anti-TNFR2 blocking mAb abolishes protective effect of Treg after allo-HCT. (A-B) [B6 x C3H]F1 female mice underwent total body irradiation (TBI) followed by transplantation with B6 BM cells plus T cells or with B6 BM cells plus T cells supplemented with HY-Tregs. HY peptide was administered at day 0, 1, 3, and 6, and mice were treated or not with anti-TNFR2 administered at day 0, 2, and 4. The experiment was performed twice, and the resulting survival (A) and clinical score (B) data were pooled and compared among the 3 groups of mice. (C-D) Experimental groups were constituted of mice grafted with B6 BM cells plus T cells treated or not with anti-TNFR2 administered at day 0, 2, and 4. The experiment was performed twice, and the resulting survival (C) and clinical score (D) data were pooled. Mice were euthanized in case of weight loss >30% of initial weight or maximal clinical grade (ie, 5/5). Kaplan-Meier survival curves were compared using log-rank test. For analysis of GVHD clinical grading curves, AUC was calculated for each mouse, and then Student t test or 1-way ANOVA with post hoc analysis was performed depending on number of comparatives. ns, nonsignificant. *P < .05; **P < .01; ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/12/10.1182_blood-2016-02-700849/4/m_1651f1.jpeg?Expires=1768318815&Signature=enhOT4JfA7yYerTxz3PKMvF1tJ6cr3~jUPyaE483HNvQvtiGPrCZM-jETXIZw-nIht0TRy4lQ2P2NQahew-9RFxWqIPC-VtnwafANpLpdk3nIfkadv2WEtqvK~gTEuxmOfgLKLxXzzTuoEOt3P41BPvL72i3gUjJnwy5SdWY~yhRGSp8E~ITC~iFOc69j9jvXSDoAzpp7szIY~T-4GgYVi7gz7tQA4tlFVwuiOlUUQE-a7O-et0loiPP508RhiLi6jB0BrMXEFNva2pLyZV-VzWvhb9cGU~KMd8rOntjprWjFU8Ikaa-OyfGE2EYd6SNwzbxe8BhCGbBGHT-rDmnQw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. TNFα/TNFR2 disruption using TNFR2-KO Tregs abolishes the protective effect of Treg after allo-HCT. [B6 x C3H]F1 female mice underwent TBI followed by transplantation with B6 BM cells plus T cells or with B6 BM cells plus T cells supplemented with HY-Tregs produced from WT B6 or from TNFR2-deficient mice in order to prevent GVHD. HY peptide was administered at day 0, 1, 3, and 6. The experiment was performed twice, and the resulting survival and clinical score data were pooled. Kaplan-Meier survival curves (A) and curves of evolution of GVHD clinical score (B) over time were compared between the 3 groups of mice. *P < .05; **P < .01; ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/12/10.1182_blood-2016-02-700849/4/m_1651f2.jpeg?Expires=1768318815&Signature=DecnuWIDCjw79vfBXpJsrJ5fmVkHmzMV7j6bbn8WFSWj7oaKjteV-e04m7eShANpXLoqhL1XBQxg9Wflj3zTdO14Gjx6msjarNnZaMj8VgOFgHFXLYSRfUMPr3gaGtiPTMMF~3El0fO-SV7wlXHcmM~d~WxNgV8ig3qdAwbgfUJ17YVZES9jJixpo3-9-BP5BVTmaKtdG8E7ToAZpseuu2LLBEtt8ZMLwlQILx6xYVJoYaqWATrztjrYiAsu-u-ZxHdK6XUMcIupY7wlLMcPRAMof0TxymfowXaqTvTbNh8crl~bO90U~5V5JX0nknB4xH1vlH2SmnGoEKdt793YlA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. TNFα/TNFR2 disruption using anti-TNFR2 blocking mAb increases inflammatory cytokine production by donor CD4 and CD8 T cells. [B6 x C3H]F1 female mice underwent TBI followed by transplantation with B6 BM cells plus T cells treated or not with anti-TNFR2 administered at day 0, 2, and 4. Mice were euthanized, and donor CD4+ and CD8+ T cells were analyzed at day 14 posttransplantation in the spleen of grafted animals. Mean absolute numbers of splenocytes and percentage of CD4+ and CD8+ donor T cells are determined as well as intracellular IFNγ and TNFα production. Each plot represents a mouse; Student t test analysis was performed to compare the anti-TNFR2 mAb effect on T cells. *P < .05; **P < .01; ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/12/10.1182_blood-2016-02-700849/4/m_1651f3.jpeg?Expires=1768318815&Signature=HbmxkHPPwJOcpPTyU9ta95zXv7t8NVQNDnjCUsyR1mzmkM6szlMVQcqXrxzqJOGBj02mMNWo7Y2MXnALkx5AsBrTwWaQyUj5Pa9Bv7yzLx4IcLfVcMFZEM0s74scbZwSIQB-F-zPt29qOY5AZ-MXYeVM47CjHS8CyMlnucMEYfhwQwUy3H3Q6DyCV6djDn55o7GQXJ37oh1syesZv3q34-fxd66YW5jbqA~6EOFFEKD7l2ECD59x~76kFaSt-8Nctz9TFPwWO2s-VUNDdjlCx88I75Wq4eIj0dg16~yzIHH~SIU0JmY6v7~M3RyNn0g36XGFrbGmPPDjiNLpF~4lhA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. TNFα/TNFR2 disruption and its effect on GVHD do not depend on the antigen specificity of therapeutic Tregs. (A-B) [B6 x C3H]F1 female mice underwent TBI followed by transplantation with (1) B6 BM cells plus 2 × 106 T cells or (2) B6 BM cells plus 2 × 106 T cells supplemented with 2 × 106 rs-Tregs or (3) BM cells plus 2 × 106 T cells collected from TNFα-deficient mice supplemented with 2 × 106 rs-Tregs. HY peptide was administered at day 0, 1, 3, and 6, and mice were treated or not with anti-TNFR2 administered at day 0, 2, and 4. The resulting survival (A) and clinical score (B) data were compared among the 3 groups of mice. Mice were euthanized in case of weight loss >30% of initial weight or maximal clinical grade (ie, 5/5). Kaplan-Meier survival curves were compared using log-rank test. For analysis of GVHD clinical grading curves, AUC was calculated for each mouse, and then 1-way ANOVA with post hoc analysis was performed. *P < .05; **P < .01; ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/12/10.1182_blood-2016-02-700849/4/m_1651f4.jpeg?Expires=1768318815&Signature=vgIAlqefzWGC6js7rv09gzdj1iJ54Nph~jFF2V-nUFpM9yyp4hjwX8XRIIhvH9LIB9X-jcSjoYMy7-9dKVaLrjYusmacxyRsMazJeWealMDhxI-2E7cuG6RUdLYyJP5zFNUPYgtWjnPWoQStS6bnXAtwYvTOgSP2BOBVTWsb2MYbYqs-BRsi71ZKj9SmPTLg6gwln9-E7h6QPkJssNCmgixfVfGTPWGWRB0G6hIDC0ozlEvJ1vzDb-GZYezfADX3~NE1Dn8KsfZDrD44-AuwVdrO4UlTEvLzN2FBaLKZ4JsG6L1StoZVBXzMKi3e1wL7lcgRnk9N9A4huqVfhhJm4A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. TNFα/TNFR2 disruption using TNFα-KO donor T cells abolishes protective effect of Treg after allo-HCT. (A-B) [B6 x C3H]F1 female mice underwent TBI followed by transplantation with B6 BM cells plus T cells or with B6 BM cells plus T cells supplemented with HY-Tregs or B6 BM cells plus T cells collected from TNFα-deficient mice instead of WT B6 mice and supplemented with HY-Tregs. HY peptide was administered at day 0, 1, 3, and 6. The experiment was performed 4 times, and the resulting survival (A) and clinical score (B) data were pooled and compared among the 3 groups of mice. (C-D) Experimental groups were constituted of mice grafted with B6 BM cells plus T cells or T cells collected from TNFα-deficient mice instead of WT B6 mice. The experiment was performed twice, and the resulting survival (C) and clinical score (D) data were pooled. Mice were euthanized in case of weight loss >30% of initial weight or maximal clinical grade (ie, 5/5). Kaplan-Meier survival curves were compared using log-rank test. For analysis of GVHD clinical grading curves, AUC was calculated for each mouse, and then Student t test or 1-way ANOVA with post hoc analysis was performed depending on number of comparatives. *P < .05; **P < .01; ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/12/10.1182_blood-2016-02-700849/4/m_1651f5.jpeg?Expires=1768318815&Signature=hXcJPJKqzD3aVOzcWFZAb1vz-RV-nNXkCMnJRs2msYA~TXBVqyjlXfQh6XfmnaFLh88Nfa5fXhz5HTwSO4SWG~Fh8uKkuqOJCRG8pdImhdHkbRdpA9vWFcIuDPdQQV6~EQRgB0vPqZMne1bq7DnBlwcpikE-9fFAoErmudDoJevYbtQFisIhikIN8-25iN9eT5p6fDZAme-oP0IxrbgjAuI5tawNwzzDdobl1MzbN7RijxD2V3vQd~slSdaoYV5HGlplFHAB~f-eb-evWd2trYyU6cHKt9n0HZLz7nmaz7b1Of4VXeKY6Tk6HDWml0tvS-qQCV8jKvZK~O2L617N-g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. TNFα/TNFR2 disruption using TNFα-KO donor T cells increases inflammatory cytokine production by donor CD4 and CD8 T cells. [B6 x C3H]F1 female mice underwent TBI followed by transplantation with B6 BM cells plus T cells or T cells collected from TNFα-deficient mice instead of WT B6 mice. Mice were euthanized, and donor CD4+ and CD8+ T cells were analyzed at day 14 posttransplantation in the spleen of grafted animals. Mean absolute numbers of splenocytes and percentage of CD4+ and CD8+ donor T cells are determined as well as intracellular IFNγ and TNFα production. Each plot represents a mouse; Student t test analysis was performed to compare mice transferred with WT or TNFα-deficient donor T cells. *P < .05; **P < .01; ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/12/10.1182_blood-2016-02-700849/4/m_1651f6.jpeg?Expires=1768318815&Signature=dxwzM6DA2U0gpRkKyMpYbKjttz6VcaYsXhEVwnbgI~ITjgIsbicPEPINZ3rpSjZ8dFeBRrYKS7G--Aqh71QN7o~Q6sznh4e3Smhk3c8UKlYDVcxZMwM-7hplKY0b6gX5w99SJYoS~~dgJ30Gh2VNbpSe-vsumjOJW5NC9~PVmhJ7xdnNwL-1hmFCs7Yi55c6SMk5lZQ51s~CaOtaFOww-XM18Cf~JCquzzIB3AOitIflKJa9PTORTttsiqE~jI1TMPMJ8e0hsbVlTRtEr4nGc0~wQ~bc0ZBDl120J1lQk6ZYYBjH7fAvYpNbntsCHVKvmGwKEkrSDdLiGzX0jMjpkQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)