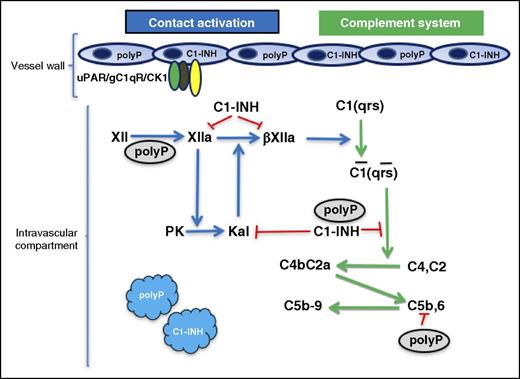
In this issue of Blood, Wijeyewickrema et al present the novel finding that polyphosphate (polyP) functions as a cofactor to C1-esterase inhibitor (C1-INH) inhibition of the enzyme C1s in the first component of the macromolecular structure of C1.1
The relationship of polyP to factor XII (FXII) activation and C1s inhibition. Zymogen FXII auto-activates to FXIIa in the presence of polyP. FXIIa is converted to a fluid-phase activator βFXIIa by plasma kallikrein (Kal). βFXIIa has the ability to convert the macromolecular complex of C1(qrs) to its enzymatically active form C1(qrs). C1s produces C3 convertase (C4bC2a) from C4, C2. C1-INH in the presence of polyP inhibits C1s. C1-INH also is known to inhibit FXIIa, βFXIIa, and Kal. Plasma kallikrein is produced by FXIIa-activating plasma prekallikrein (PK). Plasma kallikrein also has the ability to convert FXII into FXIIa by reciprocal activation on polyP (not shown). These reactions may occur in the fluid phase of the intravascular compartment, but both platelets and endothelium contain polyP and C1-INH. FXII/FXIIa also has a multiprotein receptor on endothelial cells consisting of urokinase plasminogen activator receptor (uPAR), gC1qR, and cytokeratin 1 (CK1) that could localize these reactions. These studies show the novel finding that polyP activates FXII leading to complement activation and potentiates the inhibition of C1s by C1-INH.
The relationship of polyP to factor XII (FXII) activation and C1s inhibition. Zymogen FXII auto-activates to FXIIa in the presence of polyP. FXIIa is converted to a fluid-phase activator βFXIIa by plasma kallikrein (Kal). βFXIIa has the ability to convert the macromolecular complex of C1(qrs) to its enzymatically active form C1(qrs). C1s produces C3 convertase (C4bC2a) from C4, C2. C1-INH in the presence of polyP inhibits C1s. C1-INH also is known to inhibit FXIIa, βFXIIa, and Kal. Plasma kallikrein is produced by FXIIa-activating plasma prekallikrein (PK). Plasma kallikrein also has the ability to convert FXII into FXIIa by reciprocal activation on polyP (not shown). These reactions may occur in the fluid phase of the intravascular compartment, but both platelets and endothelium contain polyP and C1-INH. FXII/FXIIa also has a multiprotein receptor on endothelial cells consisting of urokinase plasminogen activator receptor (uPAR), gC1qR, and cytokeratin 1 (CK1) that could localize these reactions. These studies show the novel finding that polyP activates FXII leading to complement activation and potentiates the inhibition of C1s by C1-INH.
PolyP potentiates the ability of C1-INH to block C1s cleavage of C4 and C2 to make C3 convertase comparable to that seen with heparin (see figure). Additionally, polyP suppresses C4d deposition on endothelial cells generated by both the classical and lectin pathways of complement. These findings suggest that this naturally occurring polyanion, unlike heparin, has several competing roles in intravascular proteolytic reactions. The polyanion heparin potentiates serpins (eg, antithrombin, C1-INH) and Kunitz-type protease inhibitors (amyloid β protein precursor or protease nexin II) to block intravascular enzymatic reactions. In the present investigation, the polyanion polyP functions like heparin to potentiate C1-INH inhibition of the classic complement pathway. However, unlike heparin, in the contact activation system, polyP has the potential to support auto-activation of zymogen FXII into an activate enzyme FXIIa (see figure). Thus, polyP is Janus-like (ie, 2-faced; under certain circumstances it is an activator of FXII and in other circumstances it is an inhibitor of C1s). Importantly, because FXII activation leads to classic complement activation by the fluid phase breakdown product of FXII-FXIIa, arginine 344-cleaved FXIIa (so-called βFXIIa), the bimodal function of polyP as an indirect activator of C1(qrs) and regulator of its product, C1s, is most interesting.
PolyP, inorganic polymers of orthophosphate units linked by phosphoanhydride bonds, are ubiquitously distributed in cells in nature in varying lengths from 30 to 800 monophosphate units in mammalian cells and thousands of units in some bacteria. PolyP are found in platelets in 60 to 100 polymers of monophosphate.2 They influence blood coagulation at the level of FXII, FV, and fibrin clot formation.2,3 The large presence of these substances in vivo may have had an evolutionary pressure on how mammalian proteins evolved.
Survival of a species in evolution is dependent upon its ability to respond to invading environmental organisms. Evolutionary pressure from bacterial infections may have forced mammals to develop a surface response system initiated by bacterial polyP polyanions. It is intelligible that FXII, a central activator of pathophysiologic blood coagulation and classic complement system, has the ability to auto-activate on an invading bacteria’s products. A system where FXII auto-activates as part of a surface defense response makes teleological sense. Likewise, the observation that platelet polyP and C1-INH colocalize in platelets suggests that these substances are positioned for their function. We would propose that a similar colocalization of polyP and C1-INH will be observed in endothelial cells. The work of Wijeyewickrema et al showing that polyP potentiates complement activation inhibition is consistent with a surface defense system that functions in a regulated manner. Additionally, it has been recently appreciated that polyP produced in cancer patients (eg, prostasomes) may be a driving force for FXII activation with increased thrombin formation and thrombosis.4 Finally, polyP itself manifests as a unique activator of FXII. A recent report that needs confirmation suggests that when polyP and FXII interact, FXII develops enzymatic activity without becoming a 2-chain protein, a so-called single chain FXIIa.5 Such a form of FXIIa could exist if there is a single cleavage of FXII at arginine 353.6 Importantly, polyP is the first polyanion that has been associated with generating such a form of FXIIa. This finding suggests that other recently recognized biologic substances (eg, RNA, DNA, collagen, and aggregated proteins) that are capable of supporting FXII auto-activation also should be studied for their potential to create novel FXIIa activated forms.
There are additional implications of the study of Wijeyewickrema et al. The fact that polyP potentiates C1-INH inhibition of C1s like heparin suggests that it may have this additional activity on its other protease target proteases, FXIIa and related forms, plasma kallikrein, and FXIa.7-9 Alternatively, it could function like heparin and protect FXIIa from inhibition by C1-INH.10 C1-INH is a heavily glycosylated protein and may be functionally influenced differently than other serpins. However, it is possible that polyP also potentiates antithrombin, α2-antiplasmin, and plasminogen activator inhibitor-1, etc inhibition of their proteases as well. Taking this line of thought further, other biologic charged surfaces (eg, RNA, DNA, and aggregated proteins) may likewise influence serpins’ inhibition of their target proteases. The implications of the study of Wijeyewickrema et al are far reaching, and may direct us to redefine the relationships between various polyanions, serpins, and target proteases. In doing so, we may observe novel mechanisms for the diversity of response so frequently seen in disease states to various pathogens. Such studies would have wide implications for our understandings of the pathophysiology of disease.
Conflict-of-interest disclosure: The author declares no competing financial interests.