Abstract
Background: A new subcutaneous formulation of rituximab (RSC) has been developed which offers reduced treatment burden for patients compared to intravenous rituximab (RIV) as well as resource savings at the treatment facility. Additionally, availability of RSC would potentially allow patients to be treated primarily IV-free with orally administered partner therapies. The Phase 3 SABRINA study (NCT01200758) investigated induction RIV or RSC plus chemotherapy followed by maintenance RIV or RSC in patients with follicular lymphoma (FL). Pharmacokinetic non-inferiority was previously reported for RSC (1400mg) compared with RIV (375mg/m2) as well as comparable efficacy and safety (Davies et al. Lancet Oncol 2014;15:343-52; Davies, et al. Haematologica 2015;100:P687). Here we present updated efficacy and safety results including overall response rates (ORR) at the end of maintenance and time to event (progression-free [PFS], overall [OS], and event-free [EFS] survival) data with a median follow-up of 37 months.
Methods: Patients with treatment-naïve CD20+ grade 1-3a FL (n=410) were randomized to receive RIV or RSC, stratified by FL International Prognostic Index score, chemotherapy regimen, and region. All patients received RIV 375mg/m2 in Cycle 1; for Cycles 2-8, patients received RIV 375mg/m2 or RSC 1400mg every 3 weeks. Patients received ≤8 cycles of CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) or 8 cycles of CVP (cyclophosphamide, vincristine and prednisone). During maintenance, patients received RIV or RSC every 8 weeks for 2 years. Non-serious adverse events (AEs) were reported for 28 days following the last dose of rituximab. Serious AEs (SAEs) were recorded for 1 year post-treatment or until the start of new anti-lymphoma treatment. SAEs considered possibly related to study treatment were recorded indefinitely.
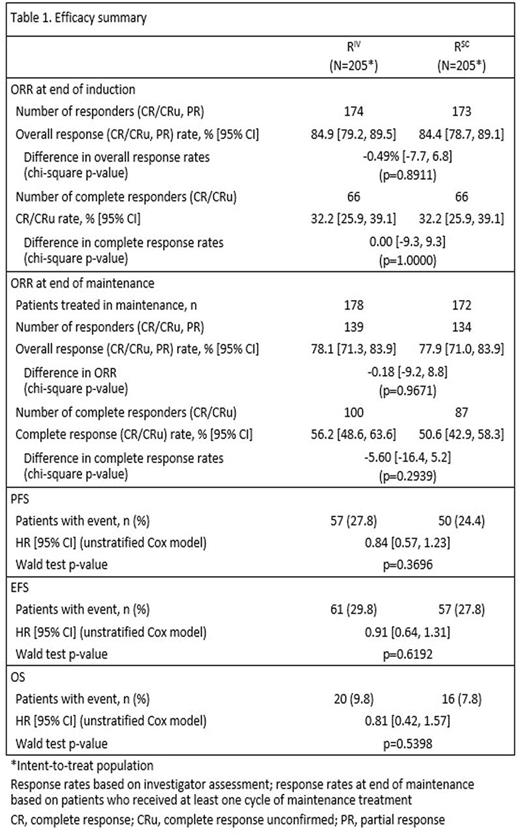
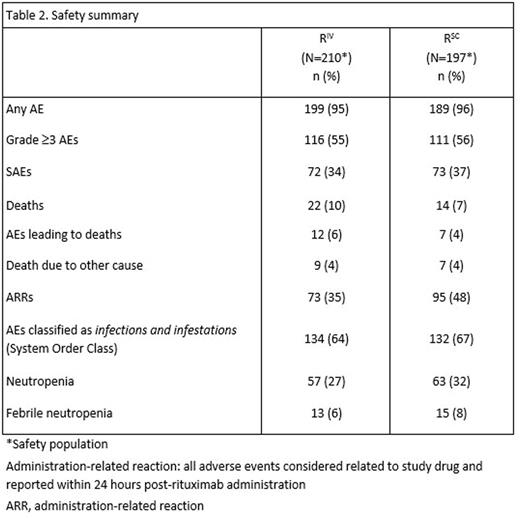
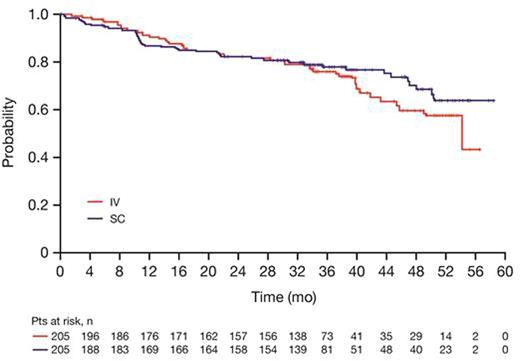
Results: Based on investigator assessments for the intent-to-treat population (RIV n=205; RSC n=205), ORR at the end of maintenance was comparable between treatment arms: (78.1%, 95% CI [71.3, 83.9] for RIV vs. 77.9%, 95% CI [71.0, 83.9] for RSC) (Table 1). Analyses of the time-to-event endpoints PFS (HR, 0.84, 95% CI [0.57, 1.23]) (Fig.1), EFS (HR, 0.91, 95% CI [0.64, 1.31), and OS (HR, 0.81, 95% CI [0.42, 1.57) showed no differences in efficacy for the SC vs. the IV formulation. In total, 407 patients received ≥1 dose of rituximab (safety population). Six RSC patients discontinued treatment after Cycle 1 (RIV in both arms) and were included in the RIV safety population (RIV n=210; RSC n=197); 86% of patients started maintenance (RIV n=178, 85%; RSC n=172, 87%). Overall, there were similar incidences of patients with ≥1 AE (95% vs. 96%), grade ≥3 AEs (55% and 56%), and SAEs (34% and 37%) for RIV vs. RSC, respectively (Table 2). The most frequent AEs reported overall (RIV vs. RSC) were neutropenia (27% vs. 32%), nausea (22% vs. 31%), constipation (26% vs. 25%), cough (13% vs. 23%), and fatigue (18% vs. 20%). The most frequently reported AEs during the maintenance phase of the study were upper respiratory tract infection (7% vs. 11%) and cough (11% vs. 12%) for RIV vs. RSC, respectively. Overall, AEs associated with B-cell depletion, including neutropenia, febrile neutropenia, and grade ≥3 infections, were balanced between the SC and IV treatment arms. The change in route of administration led to an expected higher incidence of local cutaneous reactions in the RSC arm (2% RIV vs. 23% RSC) with injection site erythema, injection site pain, and rash being most frequently reported. All reported events, except for 1 AE of injection site rash in the RSC arm at Cycle 2, were of mild or moderate intensity (grade ≤2). Incidence of local cutaneous reactions decreased over subsequent treatment cycles. At data cut-off, 36 deaths had been reported: 20 (9.8%) in the RIV arm and 16 (7.8%) in the RSC arm.
Conclusions: Overall, there were no new clinically relevant safety signals observed with RSC and the safety profile was comparable to that of RIV. Response rates as well as PFS and OS data for rituximab SC were comparable to rituximab IV and indicate that the anti-lymphoma activity of rituximab is not impaired when given subcutaneously. The availability of RSC administration over approximately 6 minutes has positive implications for patient and healthcare professional convenience, as well as healthcare resource savings, without compromising efficacy or safety.
Davies:Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Mundipharma: Honoraria; Janssen: Honoraria; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel to scientific conferences, Research Funding; GSK: Research Funding; Karyopharma: Honoraria, Research Funding; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodation, expenses, Research Funding; Gilead: Consultancy, Honoraria, Research Funding; Bayer: Research Funding; Pfizer: Research Funding. Leppä:Roche: Consultancy, Honoraria, Other: Travel expenses, Research Funding; Takeda Pharmaceuticals: Consultancy, Honoraria, Other: Travel expenses; Janssen: Consultancy, Research Funding; Mundipharma: Research Funding; CTI Bio Pharma: Consultancy; Bayer: Other: Travel expenses, Research Funding. Cotting:Roche: Employment, Equity Ownership. Veenstra:Roche: Employment. Zharkov:Roche: Employment. Dixon:Roche Products Limited: Employment, Equity Ownership. Barrett:Roche Products Ltd.: Employment, Equity Ownership. Macdonald:Roche: Consultancy, Honoraria; Lundbeck Canada: Consultancy, Honoraria; Gilead: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract