Abstract
Background: Classical Hodgkin lymphoma (cHL) is characterized by chromosome 9p24.1 alterations (including amplification), leading to overexpression of the PD-L1 and PD-L2 immune checkpoint ligands. This genetically determined dependence on the PD-1 pathway makes cHL an attractive target for PD-1 blockade with the anti-PD-1 monoclonal antibody, pembrolizumab. In the phase 1b KEYNOTE-013 study, pembrolizumab demonstrated high antitumor activity (objective response rate [ORR] of 65%) in heavily pretreated patients with cHL. KEYNOTE-087 is a phase 2 study designed to further evaluate the efficacy and safety of pembrolizumab in different subgroups of patients with relapsed/refractory (R/R) cHL.
Methods: KEYNOTE-087 (ClinicalTrials.gov, NCT02453594) is a multicenter, single-arm, multicohort phase 2 study of pembrolizumab in 3 cohorts of patients with R/R cHL: R/R cHL after autologous stem cell transplantation (ASCT) and subsequent brentuximab vedotin (BV) therapy (cohort 1); ineligibility for ASCT due to chemoresistance (no response to salvage chemotherapy) and BV therapy failure (cohort 2); and R/R cHL after ASCT but not treated with BV after ASCT (cohort 3). Patients received pembrolizumab at a fixed dose of 200 mg intravenously every 3 weeks. Response was assessed every 12 weeks according to the 2007 Revised Response Criteria for Malignant Lymphomas. The primary end point was ORR per blinded independent central review (BICR); secondary end points included ORR per investigator review (IR), complete remission rate (CRR), progression-free survival, and overall survival. All patients who received at least 1 dose of pembrolizumab were included in the analyses. Informed consent was obtained for all patients. Biomarkers included PD-L1/PD-L2 expression in formalin-fixed, paraffin-embedded tissue; flow cytometry-based evaluation of absolute and relative numbers of circulating NK cells and T-cell subsets (naive and memory T cells, activated T cells, and regulatory T cells); and gene expression using the NanoString and Illumina RNAseq platforms. The data cutoff date for these analyses was June 27, 2016.
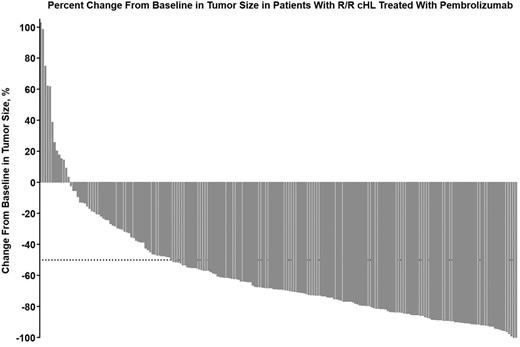
Results: Among 210 treated patients in cohorts 1 (n = 69), 2 (n = 81), and 3 (n = 60), all patients had refractory disease or relapsed HL. Of these, 98.6%, 96.3%, and 60.0% had received ≥3 prior lines of therapy, and by design 100% of patients in cohorts 1 and 2 had progressive disease after BV. 41.7% of patients received BV before ASCT in cohort 3. Per IR, ORR (95% CI) was 66.7% (54.3%-77.6%) in cohort 1 (46/69 patients), 65.4% (54.0%-75.7%) in cohort 2 (53/81 patients), and 68.3% (55.0%-79.7%) in cohort 3 (41/60 patients). The CRR was 29.0% in cohort 1, 24.7% in cohort 2, and 21.7% in cohort 3. Per BICR, the ORRs (95% CI) for each cohort were 72.5% (60.4%-82.5%), 65.4% (54.0%-75.7%), and 66.7% (53.3%-78.3%), respectively, and the CRRs were 21.7%, 22.2%, and 21.7%, respectively. A pooled analysis with hierarchical mutually exclusive categories of refractory disease (RD, n = 170) or relapse after ≥3 prior lines of therapy (Re ≥3, n = 40) was conducted across cohorts. Per BICR, ORR was 70.0% (62.5%-76.8%) in RD and 60.0% (43.3%-75.1%) in Re ≥3. Among patients with postbaseline assessment across all cohorts, 93.7% (192/205) experienced a decrease from baseline in tumor size (Figure). With a median of 9 treatment cycles, the most common treatment-related AEs (TRAEs) were pyrexia (11.0%), hypothyroidism (10.5%), diarrhea (6.7%), fatigue (6.7%), headache (6.2%), rash (6.2%), and nausea (5.7%). The most common grade 3/4 TRAEs were neutropenia (1.4%), thrombocytopenia (1.0%), and diarrhea (1.0%). Two patients died; neither death was considered to be treatment-related. At the time of analysis, 115 patients (80% of responders) had an ongoing response. Two hundred patients had evaluable pretreatment tumor tissue (archival or obtained for study) for biomarker analyses.
Conclusions: PD-1 blockade with pembrolizumab had substantial clinical activity in subsets of heavily pretreated patients with cHL. Of note, pembrolizumab induced a high ORR in chemoresistant cHL. Additional results, including duration of response per BICR and biomarker analysis, will be presented at the meeting.
Moskowitz:Celgene: Consultancy; Genentech: Consultancy; Merck: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Pharmacyclics: Research Funding. Zinzani:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; TG Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Infinity: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Bayer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Sandoz: Membership on an entity's Board of Directors or advisory committees; Millennium: Membership on an entity's Board of Directors or advisory committees. Fanale:molecular templates: Research Funding. Armand:Merck & Co., Inc.: Consultancy, Research Funding; Roche: Research Funding; Infinity: Consultancy; BMS: Consultancy, Research Funding; Otsuka: Research Funding; Tensha: Research Funding; Sequenta: Research Funding; Sigma Tau: Research Funding. Radford:Takeda: Honoraria, Research Funding; Seattle Genetics: Honoraria; Novartis: Honoraria; GlaxoSmithKline: Equity Ownership; Astra Zeneca: Equity Ownership. Ribrag:Pharmamar: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees; ArgenX: Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees; Infinity: Membership on an entity's Board of Directors or advisory committees; Esai: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Nanostring: Membership on an entity's Board of Directors or advisory committees. Vassilakopoulos:Takeda: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Genesis Pharma: Membership on an entity's Board of Directors or advisory committees. von Tresckow:Novartis: Consultancy, Other: travel grants, Research Funding; Takeda: Consultancy, Other: travel grants; Millenium: Consultancy. Shipp:Cell Signaling: Honoraria; Bayer: Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Merck, Gilead, Takeda: Other: Scientific Advisory Board. Gustafson:Merck & Co., Inc.: Employment, Other: stock, stock options. Zhang:Merck: Employment, Other: stock, stock options. Ricart:Merck & Co.: Employment; Pfizer: Equity Ownership. Balakumaran:Merck & Co.: Employment, Other: stock, stock options. Chen:Merck: Consultancy, Research Funding; Genentech: Consultancy, Speakers Bureau; Millenium: Consultancy, Research Funding, Speakers Bureau; Seattle Genetics: Consultancy, Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract