Abstract
Introduction:An attempt at tyrosine kinase inhibitor (TKI) withdrawal in deep molecular remission leads either to treatment free remission (TFR) or early molecular relapse (MolR) in chronic myeloid leukaemia (CML) patients. We hypothesise that immune responses promote sustained TFR and immunological markers may predict response following TFR attempt.
Methodology: We studied 54 CML patients (from ALLG trials CML 8, median follow-up 66 mo, and CML 10, median follow-up 24 mo) at baseline on TKI (minimum 24 mo MR4.5) and 3 mo and 6 mo following TKI discontinuation. MolR was defined as any single sample on follow-up with BCR-ABL1 >0.1% or two consecutive BCR-ABL1 positive samples at any value. Effector immune responses of CD56dim natural killer (NK) cells and NK cell receptor repertoire were characterised by flow cytometry and cytotoxic T lymphocyte (CTL) responses to leukaemia-associated-antigens (LAAs) WT1, BMI-1, PR3 and PRAME by interferon-gamma ELISPOT. Immune suppressor regulatory T cells (Treg; CD4+CD25brightCD127-FoxP3+), Granulocytic and Monocytic Myeloid-Derived Suppressor Cells (MDSCs; HLA-DR-Lin-CD11b+CD33+CD66b+CD15+ and HLA-DR-Lin-CD11b+CD33+CD66b-CD14+, respectively), Programmed cell death-1 (PD-1) expression on T cells, NK cells, B cells and Monocytes, and major B cell subsets were characterized by flow cytometry.
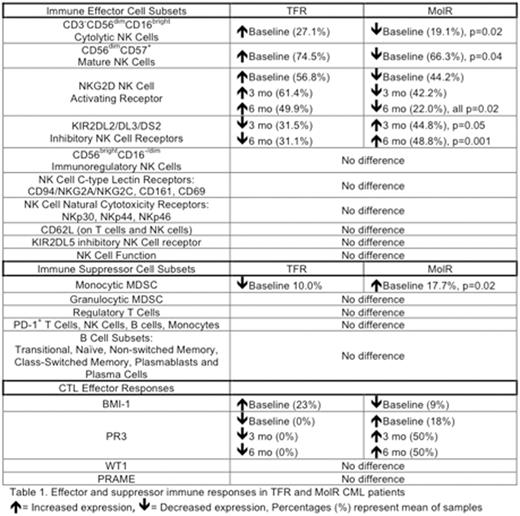
Results: TFR patients displayed increased CD3-CD56dimCD16bright cytolytic NK cells as a proportion of total lymphocytes at baseline (n=23, 27.1% ± 2.9) vs MolR (n=23, 19.1% ± 2.0, p=0.02). TFR patients displayed a more mature CD56dim CD57+ NK cell phenotype at baseline (74.5% ± 2.2 of total NK cells) vs MolR (66.3% ± 2.7, p=0.04). Extensive characterisation of NK cell receptor repertoire revealed NKG2D activating receptor expression was increased in TFR patients (baseline= 56.8% ± 3.8, 3 mo= 61.4% ± 5.0, 6 mo= 49.9% ± 5.8) vs MolR (baseline= 44.2% ± 3.7, 3 mo= 42.2% ± 5.5, 6 mo= 22.0% ± 8.3, all p=0.02). KIR2DL2/DL3/DS2-positive NK cells were increased in MolR patients at 3 and 6 mo vs TFR. (MolR; 3 mo= 44.8% ± 4.6, 6 mo= 48.8% ± 4.9. TFR; 3 mo= 31.5% ± 4.0 p=0.05, 6 mo= 31.1% ± 2.1, p=0.001).
No significant differences were observed in CD56brightCD16-/dim immunoregulatory NK cells, C-type lectin receptor expression (CD94/NKG2A/NKG2C, CD161, CD69), Natural cytotoxicity receptors (NKp30, NKp44, NKp46), CD62L (on T cells and NK cells) and KIR2DL5 expression. No difference in NK Cell-mediated K562 degranulation as a surrogate marker of NK cell function was observed between TFR and MolR patients.
Functional CTL immune responses were observed in TFR and MolR patients. BMI-1 CTL responses were increased at baseline in TFR (23%) vs MolR (9%). PR3 CTL responses were not detected in TFR at baseline, 3 mo or 6 mo (0%) vs MolR (baseline= 18%, 3 mo= 50%, 6 mo= 50%). No difference was observed in WT1 or PRAME CTLs.
Quantification of immune suppressor cell types revealed decreased Monocytic MDSCs in TFR patients at baseline (10.0% ± 2.3) vs MolR (17.7% ± 3.1, p=0.02). There was no difference in granulocytic MDSCs or Treg between TFR and MolR. No difference in PD-1 expression was observed on NK cells, T cells, B cells and Monocytes. Extensive characterisation of B cell subsets revealed no difference in TFR vs MolR (Table 1).
Conclusion: In keeping with STIM and EURO-SKI trials, a threshold level of particular NK cell subsets may be important in maintaining TFR. We found additionally that enhanced NK and CTL effector responses and decreased inhibitory NK KIR2DL2/DL3/DS2 expression, in combination with reduced monocytic MDSC may promote sustained TFR. Methods to enhance nett immune effector responses, such as mature CD56dimCD57+ NK cells and BMI-1 CTL responses or targeting inhibitory KIR may increase TFR success rates.
White:Ariad: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Ross:Novartis Pharmaceuticals: Honoraria, Research Funding; BMS: Honoraria. Hughes:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Ariad: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Honoraria. Yong:Celgene: Research Funding; BMS: Honoraria, Research Funding; Novartis: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.