Abstract
Introduction: The regulatory approval and growing clinical acceptance of the direct oral anticoagulants (DOACs: apixaban, dabigatran, edoxaban, and rivaroxaban) has challenged warfarin as the mainstay of anticoagulation for the management of venous thromboembolic disease (VTE: deep vein thrombosis and pulmonary embolism) and atrial fibrillation/flutter (Afib). As clinicians and patients choose from the expanded menu of oral anticoagulant options, it is unknown how socioeconomic variables like income, race, gender, health insurance, or marital status may influence which anticoagulant a patient utilizes.
We sought to explore if patients who stayed on warfarin differed from those who changed to a DOAC, with regard to the aforementioned socioeconomic variables. Furthermore, we desired to assess how the clinical variable of INR management, as reflected by the percent of time in therapeutic range (TTR), compared between these two groups.
Methods: The Michigan Anticoagulation Quality Improvement Initiative (MAQI2) is a multi-center collaborative registry of 6 active anticoagulation clinics across Michigan. Patients newly initiating warfarin for Afib or VTE between October 2009 and July 2016 were included. Enrollees who remained on warfarin in follow-up ("non-switchers") were compared to those that transitioned to a DOAC ("switchers") on the basis of demographics, TTR by linear interpolation, race, marital status, and insurance. Patients in each group were further analyzed in quartiles based on the median household income of their zip code of residence, derived from the 2014 U.S. Census Bureau's American Community Survey. Analyses were performed using Student's t-tests and Wilcoxon Rank-sum tests for continuous variables, and chi-square and Fisher's exact tests for categorical variables.
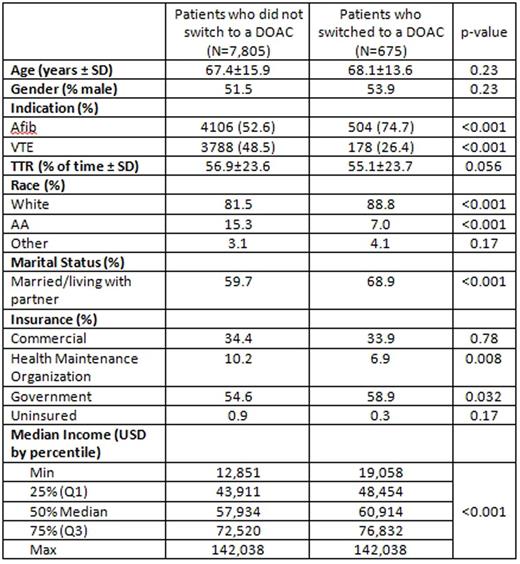
Results: 8,480 patients met the inclusion criteria, 54.4% with Afib, 45.6% with VTE, and 1.1% with both; out of this group, 675 (8%) switched from warfarin to a DOAC. There were no significant differences between switchers and non-switchers for age (mean 68.1±13.6 and 67.4±15.7, p=0.23), gender (53.9% vs. 51.5% male, p=0.23), percent TTR on warfarin (55.1% vs. 56.9%, p=0.056), or percent with commercial health insurance (33.9% vs. 34.4%, p=0.78) or uninsured (0.3% vs. 0.9%, p=0.17).
Patients were more likely to switch to DOAC therapy if they had Afib vs. VTE (10.9% vs. 4.5%, p<0.001). When comparing switchers to non-switchers, switchers were more often white race (88.8% vs. 81.5%, p<0.001), married/living with a partner (68.9% vs. 59.7%, p<0.001), and had Government Health Insurance (58.9% vs. 54.6%, p=0.032). As compared to non-switchers, switchers were less often African American (7% vs. 15.3%, p<0.001), and less often had insurance through a Health Maintenance Organization (HMO) (6.9% vs. 10.2%, p=0.008). Non-switchers more often resided in a zip code with a lower median household income compared to switchers (p<0.001).
Conclusion: While DOACs are often considered in patients who have difficulty maintaining a therapeutic INR, TTR was not predictive of changing from warfarin to a DOAC in this population. However, we found that SES factors, such as race, insurance status and income are associated with a patient's likelihood for switching to DOAC therapy vs. remaining on warfarin therapy. Further investigation into the reason for, and clinical impact of, these observed disparities in the care of our patients is needed.
Sood:Bayer: Research Funding. Kline-Rogers:Janssen: Consultancy; ACP: Consultancy; AC Forum: Membership on an entity's Board of Directors or advisory committees. Almany:Abbott: Research Funding; Kona: Consultancy; Trice Orthopedics: Consultancy; MiCardia: Consultancy; Biostar Ventures: Equity Ownership; Ablative Solutions: Equity Ownership; Boston Scientific: Research Funding. Kaatz:Pfizer: Consultancy; Bristol-Myers Squibb: Consultancy; Daiichi Sankyo: Consultancy; Janssen: Consultancy; Boehringer Ingelheim: Consultancy; Boehringer Ingelheim: Honoraria; Janssen: Honoraria; Bristol Myer Squibb: Honoraria; Pfizer: Honoraria; CSL Behring: Honoraria. Froehlich:Boehringer-Ingelheim: Membership on an entity's Board of Directors or advisory committees; Fibromuscular Disease Society of America: Research Funding; Blue Cross/Blue Shield of Michigan: Research Funding; Novartis: Consultancy; Janssen: Consultancy; Merck: Consultancy; Pfizer: Membership on an entity's Board of Directors or advisory committees. Barnes:Portolal: Consultancy; Blue Cross Blue Shield of Michigan: Research Funding; Bristol-Myers Squibb: Research Funding; Pfizer: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.