Abstract
Background
Ascorbic acid (AA) supplementation has traditionally been used in iron overloaded patients as means to increase iron chelation efficacy and replenish AA oxidized by labile iron found in those patients. The rationale leaned on AA's ability to render stored iron accessible to chelation, as found in urinary iron excretion following deferoxamine infusion. However, as AA increases labile iron redox-cycling and ensuing toxicity, we set to assess the long term benefits versus toxicity risks of the combined chelator-AA treatment.
Objectives
Perform a prospective, open-label, randomized and controlled 1 year study on thalassemia patients treated with deferasirox (DFX) in order to assess the effects of AA supplementation on: a. markers of systemic iron overload in selected organs and in plasma and b. markers of plasma labile iron (LPI) as potential contributors to oxidative stress toxicity.
Patients and Methods
Enrolment: 22 beta thalassemia major (TM) patients ≥10 years treated >2 years with DFX. Exclusion: cardiac dysfunction/arrhythmia or mT2* MRI <6 ms. Study: patients previously unexposed to AA received once-daily DFX (up to 40 mg/kg/d) with or without 125 mg AA for 1 year. All parameters were measured at baseline (BL); serum ferritin (SF) monthly, liver iron (LIC by MRI) and cardiac iron (mT2*MRI) after 1y. e-LPI (surrogate NTBI marker) and LPI (plasma redox-active labile iron marker) were assessed at BL, mo 1 & 6 by FeROS™ (Aferrix, Ltd) and fasting plasma AA at BL and EOS (fluorimetrically). Blood samples were withdrawn on the morning of transfusion day, 24 hours after last DFX (+/- AA) administration. Safety was followed using laboratory and clinical tests. AA levels were also determined in 23 healthy individuals (age and gender matched).
Results
22 TM patients were enrolled (mean age 23.5, range 10-34 y). The average dose ± SD of DFX given to all 22 patients was 38±4.5 mg/kg/d. 11 patients were randomised to receive DFX and the others with DFX supplemented with 125 mg AA (mean 2.4±0.5, range 1.9-4.2 mg/kg) for 1 year.
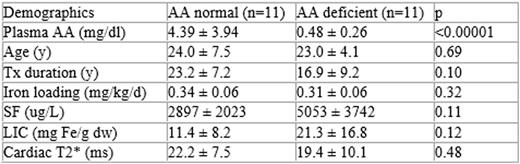
At BL, the AA levels were significantly lower in the TM group compared to controls (2.44 ± 3.38 vs 9.60± 4.36 mg/dl respectively, p<0.000001). 11 of 22 patients had AA levels >-2SD of control group whereas the other 11 patients showed normal ranges of AA. The AA deficient patients were those that showed significantly higher SF, LIC and lower mT2* at BL (Table 1).
In the DFX+AA arm, 5/11 (45%) patients had subnormal AA levels at BL but attained normal status after 1 year, as did all others on AA. Of the 5/11 (45%) DFX-treated patients that did not receive AA had normal BL AA and only 2/11 maintained normal AA status at EOS.
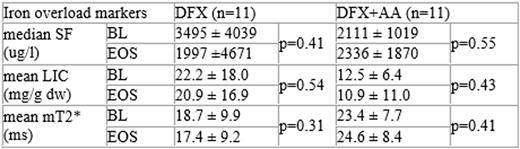
A significant correlation was obtained between BL SF, LIC and mT2* and e-LPI (r 0.49, p 0.025; r 0.57, p 0.01; r -0.43, p 0.057 respectively) but not with LPI. The changes associated with DFX alone or with AA from BL to EOS were subtle for all parameters measured (Table 2).
Importantly, eLPI and LPI remained at basal levels throughout 6 months treatment in both arms. With DFX alone, LPI were 0.34±0.30 units (mM iron) (BL) & 0.63±0.58 (6 mo); eLPI: 1.71±1.93 at BL & 2.48±3.11 (6 mo). DFX+AA: LPI were 0.33±0.46 (BL) & 0.35±0.44 (6 mo); eLPI: 2.13±1.71 (BL) & 1.78±1.51 (6 mo).
Conclusions
TM patients on long term DFX without AA supplementation showed subnormal, AA levels. This was most pronounced in TM patients with higher liver and heart iron. The addition of AA to DFX normalized the AA levels but did not increase the e-LPI and LPI during 6 mo, indicating no apparent risk of iatrogenic toxicity by AA to DFX. Moreover, AA may enhance the efficacy of DFX in cardiac and hepatic iron. The small rise in SF versus fall in LIC in the DFX+AA arm might need further exploration.
Changes in iron overload markers in patients treated with DFX or DFX+AA over 1 year
Changes in iron overload markers in patients treated with DFX or DFX+AA over 1 year
Aydinok:Novartis Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Cerus: Research Funding; Shire: Research Funding. Cabantchik:Aferrix: Consultancy, Membership on an entity's Board of Directors or advisory committees; Hinoman: Consultancy; Novartis Pharmeceuticals: Honoraria, Speakers Bureau; Apopharma: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.