Abstract
Background: The vast majority of births with sickle cell disease (SCD) occur in Africa and 90% are thought to die before the age of five. Hydroxyurea (HU) is the only drug approved by the FDA for the treatment of sickle cell anemia. Although HU is used to treat a small number of patients in Africa, cost, fear of toxicity, and lack of awareness and availability limit its use.
Methods: We prospectively assigned 31 high risk sickle cell anemia patients (hemoglobin F <8.6%, no alpha globin gene deletion, BCL11A rs1427407 GG, rs7606173 CC) to fixed low dose HU (500 mg/day) for six months and monitored the development of cytopenias and new or recurrent malaria or tuberculosis. Malaria prophylaxis was given with proguanil 200 mg po daily. Nine patients did not come regularly for monthly supplies of HU and we report on 22 patients who received medication five or six months out of the prospective six-month period. Picking up refills was the only metric for adherence to daily hydroxyurea in the study.
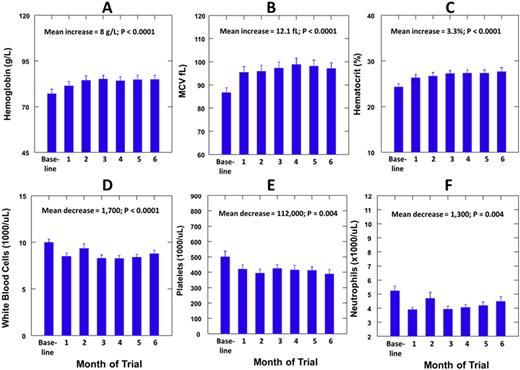
Results: The median (range) age of the patients was 24 (18-42) years and 7 (32%) were females. The median (range) dose per body weight of HU was 9.9 (7.0-13.7) mg/kg per day. Hemoglobin F was measured at baseline and during HU therapy in 15 patients and showed a mean increase of 4.7% (P=0.0001). There were mean increases in the mean corpuscular volume (MCV) of 12.1 fL, in hemoglobin of 0.8 g/L and in hematocrit of 3.3% during low dose HU therapy (P<0.0001). There were mean decreases in the white blood cell (WBC) count of 1700/uL, in absolute neutrophil count (ANC) of 1,300/uL and in platelet count of 112,000/uL. Body weight increased by a mean of 0.9 kg (P=0.030). No patient developed the predetermined toxicities of ANC <500 per uL or platelets <50,000 per uL. The lowest ANC observed was 2500 and the lowest platelet count was 76,000, which returned to >150,000 with continued therapy. One patient developed active tuberculosis while on HU therapy and another patient died of hyperhemolytic crisis two months after completing HU therapy.
Conclusion: Our results support the concept that low dose HU can be administered in the African setting with salutary features of gain in body weight, increase in hemoglobin concentration and lowering of the WBC count but absence of dangerous cytopenias. However, they raise the concern that such therapy might increase the risk of recrudescent tuberculosis. We propose that a larger prospective multicenter study including urban and rural areas should be organized to document the role of fixed low dose HU in adults with SCD in Africa.
Hemoglobin, MCV and Hematocrit increased progressively and during the first three months (A-C) and then did not change significantly during the second three months. WBC, platelets and ANC decreased progressively during the first three months (D-F) and then did not change significantly during the second three months.
Hemoglobin, MCV and Hematocrit increased progressively and during the first three months (A-C) and then did not change significantly during the second three months. WBC, platelets and ANC decreased progressively during the first three months (D-F) and then did not change significantly during the second three months.
Hsu:Centers for Medicare and Medicaid Innovation: Research Funding; EMMI Solutions: Consultancy; Sancilio: Research Funding; Astra Zeneca: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Eli Lilly: Research Funding; Gerson Lehman Group: Consultancy; Mast Therapeutics: Research Funding; Hilton Publishing: Consultancy, Research Funding; Purdue Pharma: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.