Abstract
Currently, platelet transfusions are entirely dependent on human volunteer donors, and these methods are limited by a 5-day shelf life and differences in donor/recipient immunology. In vivo, platelets are formed when bone marrow megakaryocytes (Mks) extend long, cytoplasmic projections, called proplatelets (proPLTs), into the sinusoid where shear forces accelerate PPL elongation and release platelets into circulation. Methods of in vitro platelet production have yielded less than 10 platelets/Mk (plts/Mk), compared to >1000 plts/Mk in vivo. There is a need for a clinically relevant process for in vitro platelet production, but much is unknown about what initiates and regulates PPL formation and how to maximize platelet release. We are exploring the production of functional platelet-like particles (PLPs) within a microfluidic bioreactor that utilizes shear forces on Mks to generate proPLTs and PLPs. Microfluidic devices have emerged as a valuable tool for cell culture studies. Advantages include low input cell requirements, the ability to screen multiple conditions in parallel, compatibility with time-lapse imaging, and tight control of microenvironment conditions. In addition, device fabrication is straightforward and inexpensive using soft photolithography.
Through the use of computational fluid dynamics (CFD) simulations and microfluidic device fabrication, a design - test - build methodology was used to develop a dual-flow microfluidic bioreactor system with uniform shear stress at levels similar to those found in the bone marrow niche. We are using this bioreactor to study the proPLT formation process and enhance in vitro PLP yields. Experimental studies were conducted to validate the simulations in terms of streamline profiles and flow patterns with and without cell capture. Microenvironment characteristics include but are not limited to extracellular matrix (ECM) protein coatings. Furthermore, the design of the bioreactor allows for a wide physiological shear rate range.
Our bioreactor design yields 21 ± 3 PLPs/Mk. Bioreactor-derived PLPs are shown to exhibit functional activity, as evidenced by CD41a and CD42b surface marker expression, activation (PAC1 binding and CD62P expression) in the presence of thrombin agonist, and morphological/cytoskeletal changes upon binding to fibrinogen - before and after activation. The system can be further scaled, for example, through parallelization of reactors. Furthermore, the videos and images captured within our system show that Mks make beads-on-a-string proPLTs, thick extensions that thin out into characteristic proPLTs, what appears to be shuttling of PLPs into larger bodies, and most interestingly, a burst of single PLPs upon Mk trapping. A second round of proPLT formation and PLP release by trapped Mks can be induced by increasing the flow rate through the central channel. Future studies with small-molecule modulators of the actin and microtubule cytoskeleton will help to understand the factors that regulate the initiation of proPLT formation and PLP release and improve in vitro platelet yields.
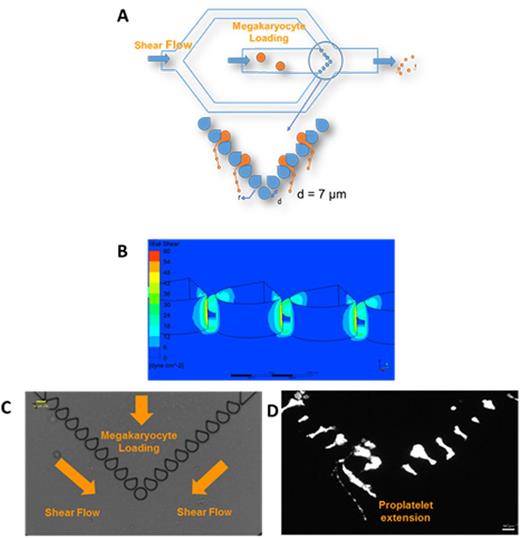
(A) Schematic for bioreactor. Two different syringe pumps are used, allowing independent flow rate changes to each channel. (B) CFD simulations of shear stress through 7-µm slits at a flow rate of 1.5 µL/min in each channel. The bioreactor generates uniform shear profiles across all slits, within and above the physiological shear stress range (1 - 4 dynes/cm2 in marrow sinusoids). (C) Bioreactor is positioned over a microscope equipped with real-time imaging and green fluorescence. The entire system is placed inside an incubator at 370C and 5% CO2. Media is perfused through the system before Mk loading. Image shows a cell-free system of the fabricated bioreactor. (D) Upon Mk (Calcein AM live stain) loading and shear exposure, Mks generate proPLTs through the slits that then get fragmented by the secondary shear flow (image at 20X, scale bar 50 μm).
(A) Schematic for bioreactor. Two different syringe pumps are used, allowing independent flow rate changes to each channel. (B) CFD simulations of shear stress through 7-µm slits at a flow rate of 1.5 µL/min in each channel. The bioreactor generates uniform shear profiles across all slits, within and above the physiological shear stress range (1 - 4 dynes/cm2 in marrow sinusoids). (C) Bioreactor is positioned over a microscope equipped with real-time imaging and green fluorescence. The entire system is placed inside an incubator at 370C and 5% CO2. Media is perfused through the system before Mk loading. Image shows a cell-free system of the fabricated bioreactor. (D) Upon Mk (Calcein AM live stain) loading and shear exposure, Mks generate proPLTs through the slits that then get fragmented by the secondary shear flow (image at 20X, scale bar 50 μm).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.