Abstract
Background: CD45 is a Receptor Protein Tyrosine Phosphatase C (PTPRC) and regulates Src Family Kinases activation in Lymphocytes. Although it is known to be absent from the platelet surface, proteomics studies prove that the CD45 c-terminal catalytic domain is present in platelets. Thus the aim of this study is to identify presence of CD45 c-terminal domain in platelets and characterize the functional implications of CD45 deficiency in platelets using a global CD45 knockout mouse.
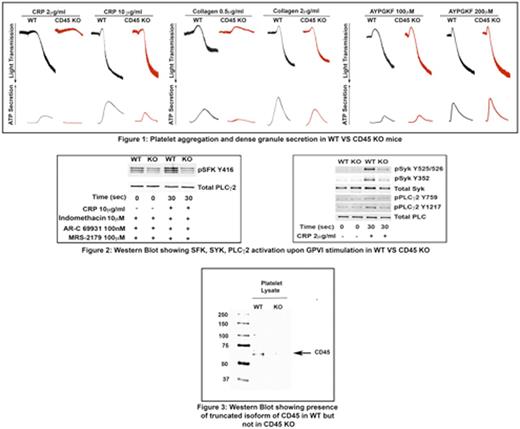
Results: Platelets from CD45-deficient mice displayed a selective impairment of aggregation and dense granule secretion mediated by the collagen receptor Glycoprotein VI. CD45 deficient mice show increased bleeding times, indicating an important role for CD45 in hemostasis. However, there was no difference observed in thrombus generation and thrombus stability using the ferric chloride-induced carotid artery injury model. Signaling downstream of the GPVI receptor, indicated by Src Family Kianse (SFK), Syk and Phospholipase C_2 (PLCg2) tyrosine phosphorylation, was also impaired. In order to establish the presence of CD45 in platelets we used an established primary antibody that recognizes the c-terminal domain of CD45. We observed that this antibody recognized a protein of approximately 65 kDa, which is the expected size of the c-terminal 1 and 2 domains of CD45, in wild type (WT) mice but not in knockout (KO)mice.
Conclusion: Thus we conclude that CD45 is expressed in platelets as a truncated form, possibly generated by proteolytic cleavage, and regulates GPVI signaling, through regulation of Src Family Kinase activation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.