Abstract
Acquired TTP is a thrombotic microangiopathy (TMA) associated with deficiency of the vonWillebrandfactor cleaving protein ADAMTS13. Atypical hemolytic uremic syndrome (aHUS) is a TMA with a genetic predisposition todysregulationin the alternative complement pathway. Causes ofaHUSinclude mutations in CD46, complement factor I (CFI), complement factor B, complement component 3 (C3), complement factor H-related 5 (CFHR5), andthrombomodulin(THBD) or secondary to Complement factor H (CFH) autoantibodies.
Treatment of these two TMAs differs. Whereas therapeutic plasma exchange (TPE), steroids, rituximab, cyclophosphamide and/or vincristine are used in TTP,aHUSrequires controlling unrestrained complement activation with eculizumab, an antibody preventing cleavage of C5.
Testing for genetic predisposition to complementdysregulationis not commonly considered in TTP. To our knowledge, only one case report has described such a genetic association (Ref: PMID# 22409250). Here we describe 3 new cases with concurrent TTP,aHUSand genetic changes in complement genes.
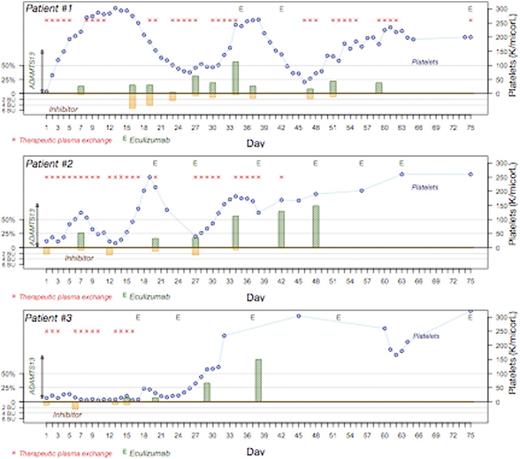
Case 1: 24y.o. African American (AA) man with XXY syndrome presented with bloody diarrhea, diffuse abdominal pain and renal dysfunction and a presumptive diagnosis ofaHUS. He was treated with eculizumab prior to transfer to our institution. On arrival to us, ADAMTS13 was low and inhibitor elevated. He initially responded to TPE but renal failure progressed andaHUS gene testing was ordered revealing a CFHR1-CFHR3 homologous deletion. Eculizumab was restarted but increased ADAMTS13 inhibitor required continued TPE, rituximab and vincristine; eculizumab was held until ADAMTS13 inhibitor was no longer detectable. At 8 months he continues on eculizumab with undetectable ADAMTS13 inhibitor and normal renal function, platelets and LDH (Figure).
Case 2: 35y.o. AA woman presented with complaints of slurred speech, left-sided numbness, and thrombocytopenia, but normal renal function. Testing revealed ADAMTS13 <5%, presence of inhibitor (2.2 BU; normal <0.4 BU) and normal complement levels. TPE and steroids were initiated, with rituximab and vincristine added due to inhibitor boosting after initial response. Despite continued treatment, clinical/laboratory parameters did not improve, C3 and C4 levels declined, andaHUS genetic mutation analysis was ordered. This revealed heterozygous missense variants in exon 11 (c.1246A>C, plle416Leu) and exon 7 (c.1135G>C,p.Val379Leu). Once platelets recovered, we treated her with one dose of eculizumab; however, subsequently her plateletsdropped as she had not yet cleared the inhibitor. We then resumed plasma exchange and added eculizumab to her treatment at a later point (figure). Her laboratory parameters have normalized and she is on eculizumab maintenance.
Case 3: 67y.o. AA woman presented with thrombocytopenia, microangiopathic hemolytic anemia, and left arm weakness. Renal function was mildly affected, with GFR ~ 30 mL/min/1.73m2. ADAMTS13 was <5% and inhibitor titer was 1.1 BU. TPE and steroids were initiated on admission, followed by rituximab, but due to lack of response after 14 days, she was given eculizumab. She had an immediate rise in platelet count after one dose and a sustained rise after the second dose. Her CBC and hemolysis parameters normalized after 4 doses. She continued treatment with eculizumab for a total of 6 months after which she opted to stop the drug. Within a month of discontinuing treatment, she developed a deep venous thrombosis and received anticoagulation for 6 months. She is now 2 years out of stopping eculizumab and has a normal CBC and LDH. Subsequent gene testing revealed the same CFHR1-CFHR3 homologous deletion identified in case 1. (Figure)
Conclusion:
We found treatment of TTP first allowed control of complementdysregulation, while treatment ofaHUSfirst did not allow control of TTP. This suggests that low ADAMTS13 levels may be the initiating factor in uncontrolled complement activation inaHUS. These cases suggest the need to test forgermlinemutations leading to abnormal complement activation in patients with refractory TTP, as they may have a dual diagnosis of both TTP andaHUSas our patients did. We therefore suggest treating the underlying TTP first, monitoring the ADAMTS13 activity and inhibitor levels to assess the clearance of the inhibitor, and initiating treatment with eculizumab once the inhibitor has cleared.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.