Abstract
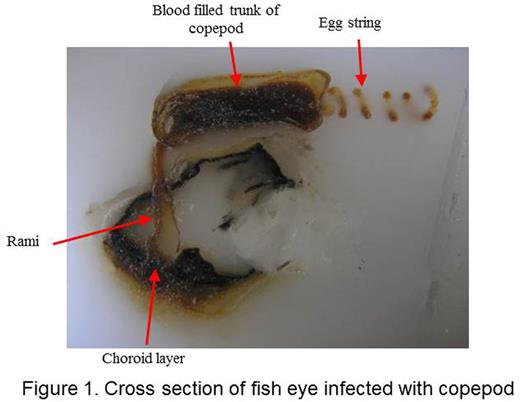
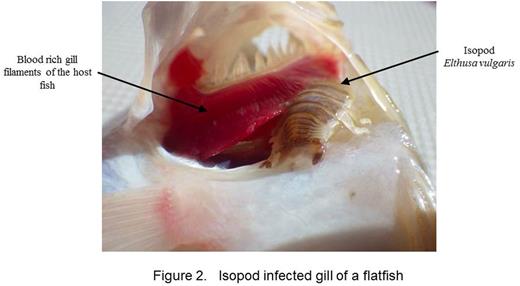
We report identification and preliminary characterization of novel proteinaceous anticoagulants from two marine crustacea, Elthusa vulgaris and Phrixocephalus cincinnatus. We also propose naming them Occipodins in honor of the college where this undergraduate research has been initiated. The organisms were collected at 25-125 meters depth off the coast of Los Angeles. Both organisms are hematophagous arthropods that anchor onto their host species and induce the extravasation of host blood into their digestive organs. Female P. cincinnatus display host specificity infecting the eyes of flatfish by anchoring to the choroid membrane and subsequently developing rami, an elaborate dendritic network, that facilitates the ingestion of extravasated blood from retinal blood vessels (Figure 1). The described species E. vulgaris is an isopod that similarly parasitizes a variety of marine fish by anchoring to the blood-rich gill filaments of their hosts (Figure 2). The accumulation of blood and attenuation of coagulation responses visually observed in the copepod trunk prompted us to initiate the identification of the anticoagulant factors in both hematophagous marine species.
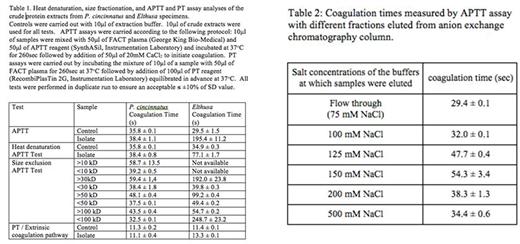
A crude cellular extract was prepared by homogenizing 10 specimens with a mortar and pestle frozen in liquid nitrogen resulting in a fine powder. The homogenate was resuspended in 15ml of 50mM Hepes buffer, pH7.4, containing 150mM NaCl and 3mM CaCl2 and mixed on a rotary shaker for 20min at room temperature. The resulting tissue suspension was centrifuged at 15,000xg 15 min and the supernatant was filtered through a 0.2 μm syringe filter. 10μl aliquots of extracts were used for all anticoagulant activity tests using FACT plasma for aPTT and PT assays on a Stago STart 4 hemostasis analyzer (Table 1). The extracts from both parasites were found to have exclusive anticoagulation activity on the intrinsic pathway (APTT assay) but no prolongation of coagulation time was observed in PT assays. Several biochemical tests were employed to further characterize the occipodins. First, size exclusion tests were performed using series of Millipore centrifugal filter units ranging from 10 to 100kD molecular weight cutoff. The size of the occipodin from P. cincinnatus was determined to be larger than 100kD while the anticoagulant isolated from Elthusa was estimated to be ~50kD. In order to identify whether these large macromolecules require a folded structure for activity we employed heat denaturation tests at 96°C for 15min. The P. cincinnatus occipodin showed substantial loss of anticoagulant activity following heat denaturation while E. vulgaris retained some residual anticoagulant activity, indicating the presence of non-proteinaceous, heat-resistant components (Table 1).
A preliminary purification procedure of the anticoagulant isolated from Elthusa was established using a DEAE anion exchange chromatography. The crude tissue extract was diluted two fold with 50mM Hepes buffer, pH7.4 and applied on a 22ml column packed with DEAE-Sephacel (GE Healthcare) anion exchange matrix equilibrated with 50mM HEPES buffer, pH 7.4, containing 75mM NaCl. To elute occipodins, a step gradient ranging from 150 to 500mM NaCl (in the same HEPES buffer) was applied, after an extensive washing step with 120ml equilibration buffer. The anticoagulant activity was detected in fractions eluted between 125mM and 500mM NaCl with main pick centered at fractions eluted with buffer containing 150mM NaCl (Table 2)
Identification of the site of inhibitory action of occipodins on the intrinsic coagulation pathway is currently in progress. In addition, further experiments will be targeted towards purification of occipodins and identification of their partial amino acid sequences. This sequence information will be used for cloning of cDNAs encoding these proteins.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.