Abstract
Direct Oral Anticoagulants (DOACs), specifically factor Xa inhibitors, have been approved for VTE treatment, VTE prophylaxis after hip and knee replacement, and stroke prevention in non-valvular atrial fibrillation. The DOACs are fast replacing warfarin for these indications due to their ease of administration, fixed dosing, and fast onset and offset of action. However, there are no specific dosing recommendations for DOACs in patient with extreme body weight because these agents were not well represented in the clinical trials, and no specific trials examining safety or efficacy in patients of extreme weight have been conducted. A recent analysis of clinical trials data by the ISTH suggests that while data is limited, the available data suggests that DOACs are safe in patients less than 120 kg at standard doses, and are not recommended in patients greater than 120 kg unless specific concentration levels are obtained to assess their efficacy.
To better understand the safety of DOACs in patients of extreme weights, we performed a retrospective analysis of a random sample of patients of low weight (<60 kg), normal weight (60-120 kg), and extreme weight (>120 kg). A random sample of 113 charts was extracted from the medical record; 34 patients were excluded due to missing data, and 79 patients were included with 27 in the <60 kg group, and 26 in both the normal and obese weight groups. Data was collected on patients from May 2011 through April 2016. Baseline patient characteristics, bleeding events, documented clot progression, and several laboratory values (hemoglobin, platelet count, creatinine and INR at commencement of anticoagulation, hemoglobin and INR at discharge or closest follow up appointment) were recorded. The outcomes assessed were rates of bleeding and clot progression.
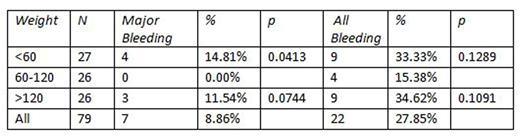
Thirty-five (44.30%) patients were male. The average age in each group was 64, 65, and 64, respectively. Forty-eight (60.76%) patients received apixaban, and the remaining patients were given rivaroxaban. Thirty-six (45.57%) patients received anticoagulation for atrial fibrillation, 26 (32.91%) had deep vein thrombosis, 13 (16.46%) had pulmonary embolism, and 4 (5.06%) had other reasons, such as peripheral arterial disease or arterial thrombus. In the low weight group, 9 patients had a documented bleeding episode (33.33%, p=0.1289), versus 4 patients in the normal weight group (15.38%) and 9 patients in the overweight group (27.85%, p=0.1091). There was no major bleeding in patients of normal weight but bleeding occurred in 4 in the underweight group 14.81%, p=.0413) and 3 in the overweight group (11.54%, p=.0744). In total, 22 of 79 patients (27.85%) had a bleeding episode. Two patients in the underweight group had clot progression (7.41%, p=0.9681), versus 2 patients in the normal weight group and no patients in the overweight group. There was no significant difference between patients who bled and those who did not bleed in creatinine, hemoglobin, platelet count or INR at the time of initiation of DOAC. We did not find a significant difference in rates of bleeding in low weight or overweight patients when compared to normal weight individuals; however there was a trend toward significance in both groups. Patients received either 5 mg bid of apixaban or 20 mg daily of rivaroxaban in both groups, therefore the difference was not attributable to different prescribing patterns. Neither group had a significant number of patients with clot progression. It is intriguing that both low weight and overweight patients had similar rates of bleeding (33.33 and 34.62%, respectively).The PK/PD data referenced by the ISTH raised the concern that overweight patients may actually be underdosed if given standard doses of DOACs. Our data, though limited by the small number of patients, poses very interesting and clinically relevant questions about DOAC use in both weight extremes: are we overdosing DOACs for underweight patients? Why are overweight patients bleeding at similar rates? We plan on formulating a prospective study to answer these questions, utilizing drug-specific peak and trough levels to ascertain sub-, supra-, and therapeutic levels in patients who bleed on DOAC therapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract