Abstract
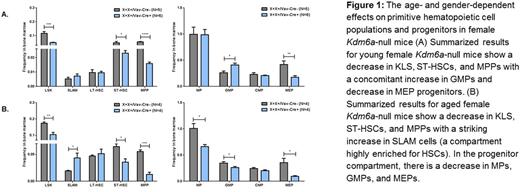
The role of the X-linked H3K27 demethylase KDM6A in normal hematopoiesis remains unclear. We generated Kdm6a conditional knockout mice (with LoxP sites flanking the 3rd exon) and crossed these mice with Vav1-Cre mice to inactivate Kdm6a in hematopoietic stem/progenitor cells. We characterized normal hematopoiesis from young (6 to 8 week old) and aged (50 to 55 week old) Kdm6a conditional KO mice. We included female and male animals. The inactivation of Kdm6a alone, both in male and female mice, results in a myeloid replating phenotype indicative of aberrant self-renewal. This is present in both young and aged mice with an increased number of colonies at week 2 in the female KO mice as compared to the hemizygous male KO mice. Interestingly, heterozygous female KO mice only acquire this abnormal replating phenotype with age. Next, we found that the genetic inactivation of Kdm6a has age- and gender-dependent effects on primitive hematopoietic cell populations and progenitors (Figure 1). The phenotype outlined in Figure 1 is specific to female Kdm6a-null mice and is not present in male hemizygous or female heterozygous mice at any time point. We went on to show that the decrease in the KLS compartment seen in the young female Kdm6a-null mice is associated with an increase in the fraction of these cells in G0/G1 along with increased apoptosis. The deletion of Kdm6a in young female homozygous mice causes mild thrombocytopenia but in aged mice is associated with anemia and thrombocytopenia. There is also a myeloid skewing with an increased number of neutrophils and a B-cell lymphopenia in the bone marrow, spleen and peripheral blood that becomes more pronounced with age in these mice. The female homozygous KO mice also have mild splenomegaly and an increased number of red blood cell precursors in the spleen.
We went on to perform a competitive transplant experiment mixing donor marrow from all cohorts of young Kdm6a conditional KO x Vav1-Cre mice (CD 45.2) with WT competitor marrow (CD 45.1 x 45.2) in a 1:1 ratio, which was then transplanted into lethally-irradiated CD45.1 recipients so that we could easily follow donor versus recipient chimerism. It has been shown that hematopoietic stem cells (HSCs) from female Kdm6a-null mice had a cell migration defect (Thieme S et al., Blood, 2013). We have now shown a decreased repopulation potential for all three cohorts of Kdm6a KO mice (homozygous and heterozygous females and hemizygous males). The finding is most pronounced in the female homozygous Kdm6a KO mice. Strikingly, and in keeping with results noted in the non-transplanted aged mice, there is a selective preservation of the donor SLAM compartment from all cohorts of the Kdm6a conditional KO mice, which is most pronounced in the female Kdm6a-null mice. The competitive repopulation disadvantage was observed when the bone marrow from primary recipients was transplanted into secondary mice. Again, however, relative preservation of the SLAM compartment was sustained. To circumvent the effects of a migration/engraftment defect of female Kdm6a-null stem cells, we repeated a competitive transplant experiment using young, female homozygous Kdm6a conditional KO x ERT2-Cre mice (and appropriate controls). After engraftment (6-weeks post-transplant), these mice were given tamoxifen to activate the Cre locus and to inactivate Kdm6a. In the bone marrow, we achieved approximately 50% floxing of the Kdm6a conditional allele 2-weeks after the last dose of drug. Again, we observed decreased repopulation potential in all lineages with the exception of the relative preservation of the SLAM compartment. We also repeated serial replating assays and showed that the floxed Kdm6a allele increased with serial replating as expected. Finally, we performed gene expression profiling via exon arrays on flow-sorted SLAM cells from aged female Kdm6a-null mice and aged controls. Hierarchical clustering revealed a clear distinction between cohorts. In sum, our data shows that female Kdm6a KO mice have a gender-specific phenotype that emerges with aging and is similar to human myelodysplastic syndrome (MDS). The female KO aged mice have an expansion of their HSCs with aberrant self-renewal, but these HSCs do not differentiate into downstream progeny as in normal hematopoiesis. As such, these mice become anemic and thrombocytopenic-but do not develop overt leukemia or die of these abnormalities.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.