Abstract
Introduction: Hypomethylating agents (HMA), including AZA, used to treat cytopenias in MDS patients (pts), can exacerbate thrombocytopenia. Such pts are usually treated with repeated platelet transfusions and AZA dose adjustments. Relieving thrombocytopenia may reduce platelet transfusion requirements and allow optimal AZA dosing. Eltrombopag is an oral TPO receptor agonist approved for the treatment of pts with chronic ITP, hepatitis C virus-related thrombocytopenia, and recurrent severe aplastic anemia. SUPPORT was a randomized, double-blind, placebo-controlled trial investigating the platelet supportive care effects of eltrombopag versus placebo in pts with intermediate (int)-1, int-2 or high-risk MDS receiving AZA.
Methods:Adult pts with no previous exposure to HMA, baseline (BL) platelets <75x109/L and int-1, int-2 or high-risk MDS by IPSS were enrolled. Pts were randomized 1:1 to eltrombopag/AZA or placebo/AZA. Pts received eltrombopag or placebo starting at 200 mg/day (100 mg/day for East Asians), adjusted by 100 mg increments (50 mg for East Asians) to a maximum 300 mg/day (150 mg/day in East Asians) - to ensure platelet counts remained sufficient to avoid platelet transfusions and bleeding events - for as long as pts received AZA (75 mg/m2 sc once daily for 7 days, every 28 days). Treatment could continue as long as benefit was derived or until disease progression, unacceptable toxicity or death. Primary endpoint was proportion of platelet-transfusion independent pts during AZA cycles 1-4. Key secondary endpoints included overall survival (OS) and disease progression. Adverse events (AEs) were recorded from the first dose of treatment until completion of the 4-week follow-up period following discontinuation of study treatment. Following a planned interim assessment, an independent data monitoring committee recommended stopping the study prematurely because outcomes crossed the pre-defined futility threshold and for safety reasons. Here we report the primary analyses from all randomized pts at the time of trial termination.
Results:356 pts (median age 70 [range 24-89] years) received eltrombopag (n=179; n=64 int-1, n=77 int-2, n=38 high-risk) or placebo (n=177; n=61 int-1, n=83 int-2, n=33 high-risk). 29 (16%) eltrombopag and 37 (21%) placebo pts were platelet-transfusion dependent at BL. Median time on AZA plus eltrombopag or placebo was 83 (range 1-477) vs 149 (8-503) days. Most common reasons (≥10%) for treatment discontinuation on eltrombopag or placebo, respectively, were study termination (32 vs 44%), AZA discontinuation (30 vs 26%) and AEs (22 vs 14%). Median eltrombopag or placebo doses were 113 (range 60-148) vs 122 (81-147) mg/day in East Asians and 200 (65-293) vs 262 (107-316) mg/day in non-East Asians.
At an interim analysis (n=147 evaluable pts), 13/79 (16%) eltrombopag and 27/68 (40%) placebo pts were platelet-transfusion independent [OR: 0.25, 95% CI: 0.11, 0.61; one-sided P=1.000], crossing the pre-defined futility boundary. At premature (primary and final analysis) study termination (n=356), 28/179 (16%) eltrombopag and 55/177 (31%) placebo pts were platelet-transfusion independent during the first 4 cycles of AZA (OR: 0.37, 95% CI: 0.21, 0.65; one-sided P=1.000).
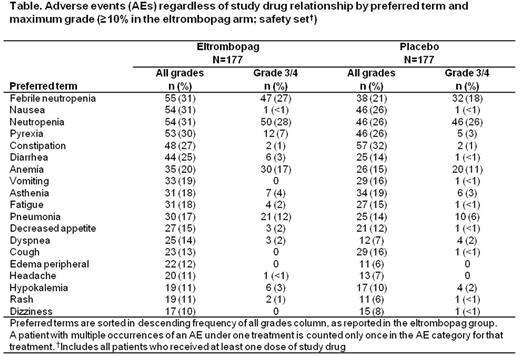
At study closure, 57 (32%) and 51 (29%) pts had died in the eltrombopag and placebo arms, respectively, with 33 (19%) and 29 (16%) deaths within 30 days after end of treatment; the main causes of death (≥10%) were disease under study (16 vs 12%) and sepsis (10 vs 7%). Disease progression (investigator-assessed) had occurred in 38 (21%) and 30 (17%) pts in the eltrombopag and placebo groups, respectively, at the time of study discontinuation. The most common AEs for eltrombopag are summarized (Table).
Conclusions: In contrast to published reports on eltrombopag monotherapy inducing platelet transfusion independence in MDS pts, eltrombopag given concomitantly with AZA was inferior to placebo/AZA. There was no difference in overall deaths between the two groups. A complete assessment of disease progression, including AML progression at the time of study termination, is in progress. Pharmacodynamic antagonism between AZA and eltrombopag is proposed as one potential explanation for these results; further analyses and preclinical studies are ongoing to determine a possible mechanism of drug-drug interaction and the impact of drug sequencing on this potential association.
Dickinson:GlaxoSmithKline: Consultancy, Research Funding. Fenaux:Celgene, Janssen, Novartis, Astex, Teva: Research Funding; Celegene, Novartis, Teva: Honoraria. Mittleman:Celgene, Novartis, Janssen: Speakers Bureau; Novartis, Pfizer, Janssen, Roche: Consultancy. Portella:Novartis: Employment. Burgess:Novartis: Employment. Platzbecker:Celgene Corporation: Honoraria, Research Funding; TEVA Pharmaceutical Industries: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Janssen-Cilag: Honoraria, Research Funding; Novartis: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.